Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00WDY
|
||||
| Former ID |
DIB007364
|
||||
| Drug Name |
BMS-903452
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Phase 1 | [523251] | ||
| Company |
Bristol-Myers Squibb Co
|
||||
| Structure |
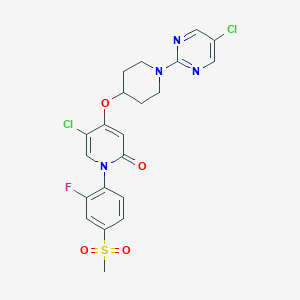
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Glucose-dependent insulinotropic receptor | Target Info | Modulator | [532950] | |
| KEGG Pathway | cAMP signaling pathway | ||||
| Insulin secretion | |||||
| WikiPathways | Incretin Synthesis, Secretion, and Inactivation | ||||
| References | |||||
| Ref 523251 | ClinicalTrials.gov (NCT01240980) Safety Study of BMS-903452 in Healthy Subjects (Panel 1-7) & Relative Bioavailability of the Crystalline and Amorphous Forms of BMS-903452 [Panels 4, 6, 11 & 12(Part A)], and Subjects With Type 2 Diabetes Mellitus (Part B). U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

