Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0UX6Z
|
||||
| Drug Name |
Heparin Sodium
|
||||
| Synonyms |
Heparin Sodium; Heparin Lock Flush; Heparin Sodium; Heparin Sodium 1,000 Units And Sodium Chloride 0.9% In Plastic Container; Heparin Sodium 1,000 Units In Dextrose 5% In Plastic Container; Heparin Sodium 1,000 Units In Sodium Chloride 0.9% In Plastic Container; Heparin Sodium 10,000 Units And Dextrose 5% In Plastic Container; Heparin Sodium 10,000 Units In Dextrose 5%; Heparin Sodium 10,000 Units In Dextrose 5% In Plastic Container; Heparin Sodium 10,000 Units In Sodium Chloride 0.45%; Heparin Sodium 10,000 Units In Sodium Chloride 0.9%; Heparin Sodium 12,500 Units In Dextrose 5%; Heparin Sodium 12,500 Units In Dextrose 5% In Plastic Container; Heparin Sodium 12,500 Units In Sodium Chloride 0.45% In Plastic Container; Heparin Sodium 12,500 Units In Sodium Chloride 0.9%; Heparin Sodium 2,000 Units And Sodium Chloride 0.9% In Plastic Container; Heparin Sodium 2,000 Units In Dextrose 5% In Plastic Container; Heparin Sodium 2,000 Units In Sodium Chloride 0.9% In Plastic Container; Heparin Sodium 20,000 Units And Dextrose 5% In Plastic Container; Heparin Sodium 20,000 Units In Dextrose 5% In Plastic Container; Heparin Sodium 25,000 Units And Dextrose 5% In Plastic Container; Heparin Sodium 25,000 Units In Dextrose 5%; Heparin Sodium 25,000 Units In Dextrose 5% In Plastic Container; Heparin Sodium 25,000 Units In Sodium Chloride 0.45% In Plastic Container; Heparin Sodium 25,000 Units In Sodium Chloride 0.9%; Heparin Sodium 25,000 Units In Sodium Chloride 0.9% In Plastic Container; Heparin Sodium 5,000 Units And Sodium Chloride 0.9% In Plastic Container; Heparin Sodium 5,000 Units In Dextrose 5% In Plastic Container; Heparin Sodium 5,000 Units In Sodium Chloride 0.45%; Heparin Sodium 5,000 Units In Sodium Chloride 0.9%; Heparin Sodium 5,000 Units In Sodium Chloride 0.9% In Plastic Container; Heparin Sodium In Plastic Container; Heparin Sodium Preservative Free; Lipo-hepin; Liquaemin Lock Flush; Liquaemin Sodium; Liquaemin Sodium Preservative Free; Panheprin; Sodium Heparin
|
||||
| Indication | Coagulation [ICD10:I80-I82] | Approved | [551871] | ||
| Therapeutic Class |
Cardiovascular Agents
|
||||
| Company |
Hospira Inc
|
||||
| Structure |
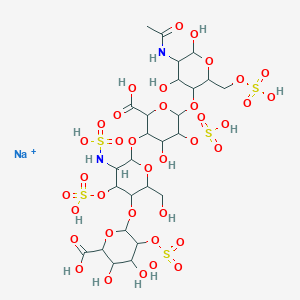
|
Download2D MOL |
|||
| Formula |
C26H41NO34S4
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Antithrombin-III | Target Info | Modulator | [556264] | |
| KEGG Pathway | Complement and coagulation cascades | ||||
| PANTHER Pathway | Blood coagulation | ||||
| Pathway Interaction Database | Glypican 1 network | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

