Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T10265 | ||||
| Target Name | Phosphodiesterase 4B | ||||
| Synonyms | DPDE4; PDE32; Type 4 cyclic adenosine monophosphate phosphodiesterase (type 4 PDE); Type 4B cAMP phosphodiesterase; PDE4B | ||||
| Target Type | Clinical Trial | ||||
| Gene Name | PDE4B | ||||
| Biochemical Class | Phosphoric diester hydrolases | ||||
| UniProt ID | PDE4B_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
| Disease | Asthma | ||||
| Example drug | SOTB07 | Phase 3 | [524380], [1572592] | ||
| Tissue | Nasal and bronchial airway | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.06 Z-score: 0.11 P-value: 2.60E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Atopic dermatitis | ||||
| Example drug | TA-7906 | Phase 2 | [548322], [1572592] | ||
| Tissue | Skin | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -1.75E-03 Z-score: -2.79E-03 P-value: 4.84E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | LIRIMILAST | Phase 2 | [551720], [1572592] | ||
| Tissue | Lung tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.17 Z-score: 0.21 P-value: 1.56E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
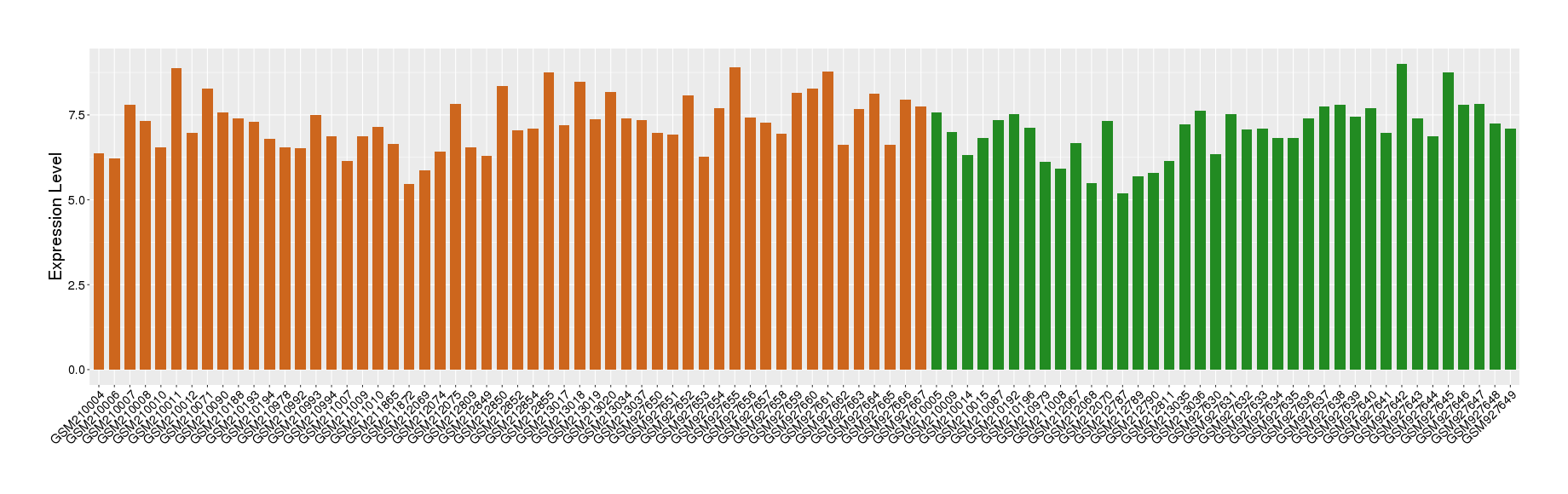
|
|||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | LIRIMILAST | Phase 2 | [551720], [1572592] | ||
| Tissue | Small airway epithelium | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.02 Z-score: 0.03 P-value: 2.90E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Psoriasis | ||||
| Example drug | MK-0873 | Phase 2 | [521664], [1572592] | ||
| Tissue | Skin | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.48 Z-score: -0.51 P-value: 4.33E-11 |
||||
| Level of differential expression between the patients in the disease section of the tissue and the patients in the normal section of the tissue adjacent to the disease section | Fold-change: 0.97 Z-score: 3.04 P-value: 4.37E-36 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Parkinson's disease | ||||
| Example drug | AVE-8112 | Phase 1 | [524229], [1572592] | ||
| Tissue | Substantia nigra tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.13 Z-score: -0.30 P-value: 2.75E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
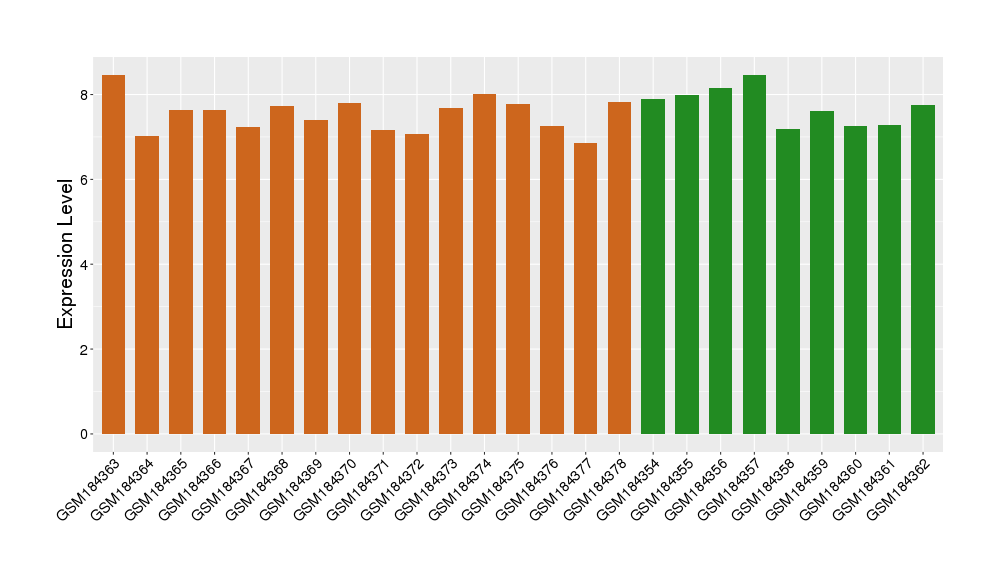
|
|||||
| Disease | Multiple sclerosis | ||||
| Example drug | Daxalipram | Discontinued in Phase 2 | [547377], [1572592] | ||
| Tissue | Spinal cord | ||||
| Level of differential expression between the patients in the disease section of the tissue and the patients in the normal section of the tissue adjacent to the disease section | Fold-change: 0.27 Z-score: 0.42 P-value: 6.29E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section

|
|||||
| Disease | Rheumatoid arthritis | ||||
| Tissue | Synovial tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 1.93 Z-score: 5.67 P-value: 1.97E-08 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Septic shock | ||||
| Tissue | Whole blood | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -1.00 Z-score: -1.22 P-value: 2.94E-27 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
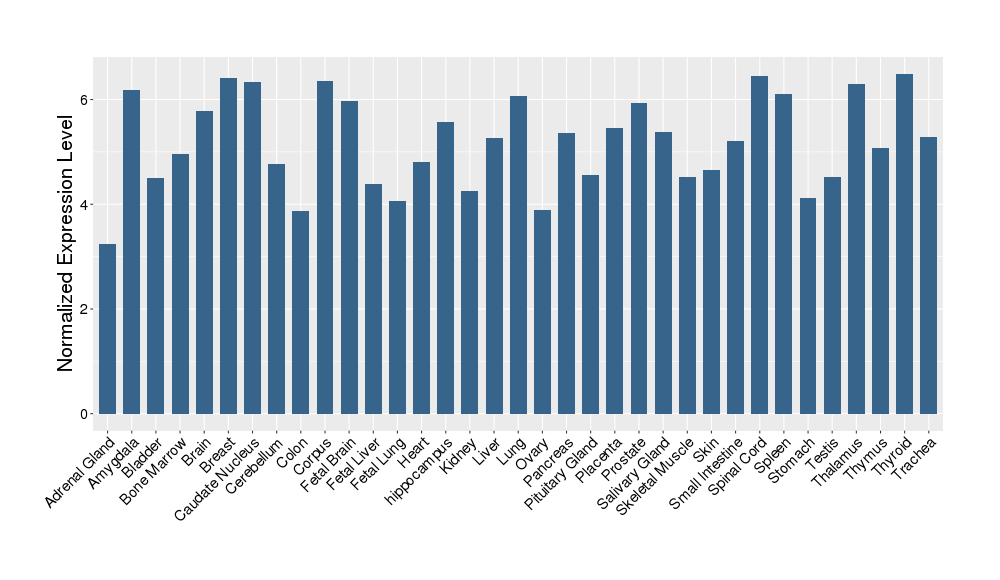
|
|||||
| Reference | |||||
| Ref 547377 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015672) | ||||
| Ref 548322 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024588) | ||||
| Ref 551720 | A novel phosphodiesterase 4 inhibitor template. Expert Opinion on Therapeutic Patents,2003, 13(6), 929-933. | ||||
| Ref 521664 | ClinicalTrials.gov (NCT00132730) An Investigational Drug Study In Patients With COPD (Chronic Obstructive Pulmonary Disease) (MK-0873-005). U.S. National Institutes of Health. | ||||
| Ref 524229 | ClinicalTrials.gov (NCT01803945) A Multiple Ascending Dose Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of AVE8112 in Patients With Parkinson's Disease. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

