Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01AYJ
|
|||
| Former ID |
DAP000753
|
|||
| Drug Name |
Terbinafine
|
|||
| Synonyms |
Bramazil; Lamasil; TerbiFoam; Terbina; Lamisil AT; Lamisil Tablet; Ternbinafine HCl; Lamasil (TN); Lamisil (TN); SF 86-327; Terbisil (TN); Zabel (TN); SF-86-327; Terbinafine (USAN/INN); Terbinafine [USAN:BAN:INN]; Lamisil, Terbinex, Corbinal, Zabel, Terbinafine; Terbinafine, SF-86-327, Lamisil, TBNF; (2E)-N,6,6-trimethyl-N-(1-naphthylmethyl)-2-hepten-4-yn-1-amine; (2E)-N,6,6-trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine; (E)-N,6,6-trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine; (E)-N-(6,6-Dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalene methanamine; (E)-N-(6,6-Dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalenemethylamine; (E)-N-(6,6-dimethyl-2-heptenynyl)-N-methyl-1-naphthalenementhamin hydrochloride
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Fungal infection [ICD-11: 1F29-1F2F; ICD-10: B35-B49; ICD-9: 110-118] | Approved | [1] | |
| Onychomycosis [ICD-11: EE12.1] | Phase 2 | [2] | ||
| Therapeutic Class |
Antifungal Agents
|
|||
| Company |
Celtic Pharma
|
|||
| Structure |
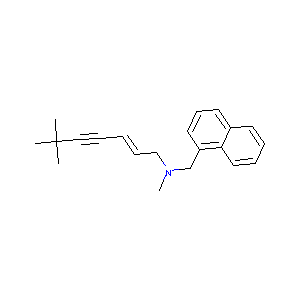 |
Download2D MOL |
||
| Formula |
C21H25N
|
|||
| Canonical SMILES |
CC(C)(C)C#CC=CCN(C)CC1=CC=CC2=CC=CC=C21
|
|||
| InChI |
1S/C21H25N/c1-21(2,3)15-8-5-9-16-22(4)17-19-13-10-12-18-11-6-7-14-20(18)19/h5-7,9-14H,16-17H2,1-4H3/b9-5+
|
|||
| InChIKey |
DOMXUEMWDBAQBQ-WEVVVXLNSA-N
|
|||
| CAS Number |
CAS 91161-71-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10279, 606886, 3206272, 7849434, 7980758, 8653314, 14800096, 26754460, 29215261, 32248932, 46508519, 48416599, 49995731, 50593794, 53790235, 56313213, 57288850, 57409064, 85279511, 90452133, 92308716, 92711333, 93166983, 93625488, 103215995, 103929637, 104253338, 105999123, 110525170, 124757457, 124766229, 124899382, 125093510, 125164261, 125325631, 126571952, 126630967, 126657418, 126664777, 126831911, 131330035, 134338039, 135014005, 135692246, 137003885, 142367778, 144205144, 162179180, 164786802, 170465174
|
|||
| ChEBI ID |
CHEBI:9448
|
|||
| ADReCS Drug ID | BADD_D01241 ; BADD_D02160 ; BADD_D02161 | |||
| SuperDrug ATC ID |
D01AE15; D01BA02
|
|||
| SuperDrug CAS ID |
cas=091161716
|
|||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Candida Squalene epoxidase (Candi ERG1) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Safety and tolerability of oral antifungal agents in the treatment of fungal nail disease: a proven reality. Ther Clin Risk Manag. 2005 Dec;1(4):299-306. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

