Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03HYX
|
|||
| Former ID |
DAP001042
|
|||
| Drug Name |
Mometasone
|
|||
| Synonyms |
Elocom; Mometasona; Mometasonum; Mometasona [Spanish]; Mometasonum [Latin]; Asmanex (TN); Elocon (TN); Mometasone (INN); Nasonex (TN); Twisthaler (TN); (+)-Mometasone; (8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloroacetyl)-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; (9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloroacetyl)-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; 9,21-dichloro-11beta,17-dihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Skin allergy [ICD-11: 4A82; ICD-9: 995.3] | Approved | [1] | |
| Therapeutic Class |
Antiinflammatory Agents
|
|||
| Company |
Schering-Plough
|
|||
| Structure |
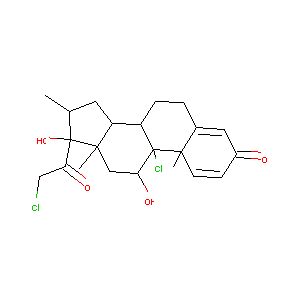 |
Download2D MOL |
||
| Formula |
C22H28Cl2O4
|
|||
| Canonical SMILES |
CC1CC2C3CCC4=CC(=O)C=CC4(C3(C(CC2(C1(C(=O)CCl)O)C)O)Cl)C
|
|||
| InChI |
1S/C22H28Cl2O4/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,24)17(26)10-20(16,3)22(12,28)18(27)11-23/h6-7,9,12,15-17,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1
|
|||
| InChIKey |
QLIIKPVHVRXHRI-CXSFZGCWSA-N
|
|||
| CAS Number |
CAS 105102-22-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:6970
|
|||
| ADReCS Drug ID | BADD_D01490 ; BADD_D01491 ; BADD_D01492 | |||
| SuperDrug ATC ID |
D07AC13; D07XC03; R01AD09; R03BA07
|
|||
| SuperDrug CAS ID |
cas=105102225
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Veillonellales | ||||
|
Studied Microbe: Veillonella parvula
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Veillonella parvula was decreased by Mometasone furoate (adjusted p-values: 1.46E-05). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glucocorticoid receptor (NR3C1) | Target Info | Agonist | [3] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| NetPath Pathway | IL2 Signaling Pathway | |||
| TCR Signaling Pathway | ||||
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | |||
| Signaling events mediated by HDAC Class II | ||||
| FOXA2 and FOXA3 transcription factor networks | ||||
| Glucocorticoid receptor regulatory network | ||||
| Regulation of Androgen receptor activity | ||||
| AP-1 transcription factor network | ||||
| Reactome | BMAL1:CLOCK,NPAS2 activates circadian gene expression | |||
| WikiPathways | Serotonin Receptor 4/6/7 and NR3C Signaling | |||
| SIDS Susceptibility Pathways | ||||
| Nuclear Receptors Meta-Pathway | ||||
| Endoderm Differentiation | ||||
| Hair Follicle Development: Cytodifferentiation (Part 3 of 3) | ||||
| Adipogenesis | ||||
| Circadian Clock | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 2 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 3 | Mometasone furoate is a less specific glucocorticoid than fluticasone propionate. Eur Respir J. 2002 Dec;20(6):1386-92. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

