Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04KJO
|
|||
| Former ID |
DAP000247
|
|||
| Drug Name |
Formoterol
|
|||
| Synonyms |
Foradile; Formoterol tartrate; Oxeze Turbuhaler Foradil; MOLI000351; Atimos (TN); Atock (TN); Foradil (TN); Foradile (TN); Formoterol (INN); Modulite (TN); Oxeze (TN); Oxis (TN); Perforomist (TN); Formoterol, ((R*,R*)-(+-)-)-isomer; N-[2-hydroxy-5-[1-hydroxy-2-[1-(4-methoxyphenyl)propan-2-ylamino]ethyl]phenyl]formamide; N-[2-hydroxy-5-(1-hydroxy-2-{[1-(4-methoxyphenyl)propan-2-yl]amino}ethyl)phenyl]formamide; N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(2S)-1-(4-methoxyphenyl)propan-2-yl]amino]ethyl]phenyl]formamide; (+-)-2'-Hydroxy-5'-((RS)-1-hydroxy-2-(((RS)-p-methoxy-alpha-methylphenethyl)amino)ethyl)formanilide; 3-formylamino-4-hydroxy-alpha-(N-1-methyl-2-p-methoxyphenethylaminomethyl)benzyl alcohol.hemifumarate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8; ICD-9: 493] | Approved | [1], [2] | |
| Therapeutic Class |
Sympathomimetics
|
|||
| Company |
Norvatis Phamaceuticals Corporation
|
|||
| Structure |
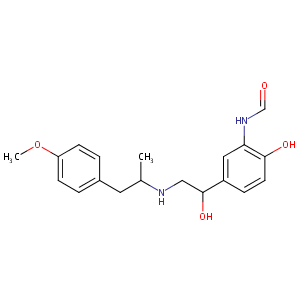 |
Download2D MOL |
||
| Formula |
C19H24N2O4
|
|||
| Canonical SMILES |
CC(CC1=CC=C(C=C1)OC)NCC(C2=CC(=C(C=C2)O)NC=O)O
|
|||
| InChI |
1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22)
|
|||
| InChIKey |
BPZSYCZIITTYBL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 73573-87-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10007, 5643479, 8152174, 14875897, 29222544, 46507099, 50125837, 50940891, 53790377, 57321784, 85209082, 92712339, 96024684, 103837996, 104303431, 117625425, 125564207, 126625648, 126656778, 126670908, 127551521, 134337561, 135610485, 136352502, 137070085, 142452394, 144224272, 152100598, 160964320, 162178864, 163329494, 176251370, 178100459, 179148573, 196111556, 198992021, 206246320, 221672992, 223400949, 223680169, 223781951, 226683973, 240785438
|
|||
| ChEBI ID |
CHEBI:63082
|
|||
| ADReCS Drug ID | BADD_D00958 ; BADD_D00959 ; BADD_D00960 | |||
| SuperDrug ATC ID |
R03AC13
|
|||
| SuperDrug CAS ID |
cas=043229807
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor beta-2 (ADRB2) | Target Info | Agonist | [3], [4], [5] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Endocytosis | ||||
| Adrenergic signaling in cardiomyocytes | ||||
| Salivary secretion | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Beta2 adrenergic receptor signaling pathway | ||||
| Pathway Interaction Database | Arf6 trafficking events | |||
| Arf6 signaling events | ||||
| Reactome | Adrenoceptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Vitamin D Receptor Pathway | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3465). | |||
| REF 2 | Long duration of airway but not systemic effects of inhaled formoterol in asthmatic patients. Respir Med. 2008 Mar;102(3):449-56. | |||
| REF 3 | Long-acting beta2-agonists for chronic obstructive pulmonary disease patients with poorly reversible airflow limitation. Cochrane Database Syst Rev. 2002;(3):CD001104. | |||
| REF 4 | Tocolytic effects of a long-acting beta2-adrenoceptor agonist, formoterol, in rats. J Pharm Pharmacol. 2000 Nov;52(11):1417-23. | |||
| REF 5 | Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2000;(2):CD001104. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

