Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05AHE
|
|||
| Former ID |
DAP000978
|
|||
| Drug Name |
Methylergonovine
|
|||
| Synonyms |
Basofortina; Ergotyl; Metenarin; Methergen; Methergin; Methergine; Methylergobasin; Methylergobasine; Methylergobrevin; Methylergometrin; Methylergometrine; Methylergometrinum; Methylergonovin; Metilergometrina; Metilergometrinio; Partergin; Ryegonovin; Lysergic acid butanolamide; Methylergometrine maleate; Metilergometrina [DCIT]; ME 277; Ergotyl (TN); Methergine (TN); Methylergometrine (INN); Methylergometrine [INN:BAN]; Methylergometrinum [INN-Latin]; Metilergometrinio [INN-Spanish]; Spametrin-M; D-Lysergic acid-dl-hydroxybutylamide-2; N-(alpha-(Hydroxymethyl)propyl)-D-lysergamide; D-Lysergic acid-(+)-butanolamide-(2); (8beta)-N-[(1S)-1-(hydroxymethyl)propyl]-6-methyl-9,10-didehydroergoline-8-carboxamide; 9,10-Didehydro-N-(1-(hydroxymethyl)propyl)-6-methylergoline-8-carboxamide; 9,10-Didehydro-N-(alpha-(hydroxymethyl)propyl)-6-methyl-ergoline-8-beta-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Spontaneous abortion [ICD-11: JA00.0] | Approved | [1] | |
| Therapeutic Class |
Oxytocics
|
|||
| Company |
Edison Therapeutics Llc
|
|||
| Structure |
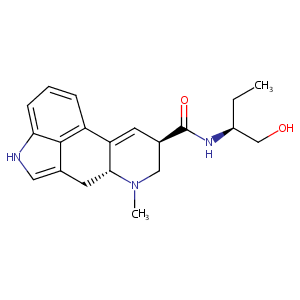 |
Download2D MOL |
||
| Formula |
C20H25N3O2
|
|||
| Canonical SMILES |
CCC(CO)NC(=O)C1CN(C2CC3=CNC4=CC=CC(=C34)C2=C1)C
|
|||
| InChI |
1S/C20H25N3O2/c1-3-14(11-24)22-20(25)13-7-16-15-5-4-6-17-19(15)12(9-21-17)8-18(16)23(2)10-13/h4-7,9,13-14,18,21,24H,3,8,10-11H2,1-2H3,(H,22,25)/t13-,14+,18-/m1/s1
|
|||
| InChIKey |
UNBRKDKAWYKMIV-QWQRMKEZSA-N
|
|||
| CAS Number |
CAS 113-42-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7979943, 8155761, 11335532, 11360771, 11363157, 11365719, 11368281, 11371623, 11376443, 11461743, 11484282, 11488429, 11490318, 11494077, 14777917, 26751766, 29226945, 46507746, 47216702, 47515240, 47588916, 47662201, 47885332, 47885333, 48110372, 48184922, 48334408, 48416242, 49699341, 49965412, 50104453, 57324864, 75350935, 85788317, 85789357, 90341291, 92729787, 96024895, 104171343, 104317266, 123085521, 123109975, 124750004, 124882912, 124882913, 127346532, 127346533, 134338249, 134973870, 135650591
|
|||
| ChEBI ID |
CHEBI:92607
|
|||
| ADReCS Drug ID | BADD_D01428 ; BADD_D01429 ; BADD_D02417 | |||
| SuperDrug ATC ID |
G02AB01
|
|||
| SuperDrug CAS ID |
cas=000113428
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Dopamine D1 receptor (D1R) | Target Info | Antagonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Gap junction | ||||
| Dopaminergic synapse | ||||
| Parkinson's disease | ||||
| Cocaine addiction | ||||
| Amphetamine addiction | ||||
| Morphine addiction | ||||
| Alcoholism | ||||
| Panther Pathway | Dopamine receptor mediated signaling pathway | |||
| Pathwhiz Pathway | Dopamine Activation of Neurological Reward System | |||
| Reactome | Dopamine receptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Hypothetical Network for Drug Addiction | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Genes and (Common) Pathways Underlying Drug Addiction | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Reinforcement in an in vitro analog of appetitive classical conditioning of feeding behavior in Aplysia: blockade by a dopamine antagonist. Learn Mem. 2005 May-Jun;12(3):216-20. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

