Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08HIH
|
|||
| Former ID |
DIB001036
|
|||
| Drug Name |
ERB-196
|
|||
| Synonyms |
WAY-196; WAY-202196; Estrogen receptor beta agonist (oral, inflammation/sepsis), Wyeth
Click to Show/Hide
|
|||
| Indication | Inflammatory bowel disease [ICD-11: DD72; ICD-10: K50-K52] | Discontinued in Phase 1 | [1] | |
| Company |
Wyeth
|
|||
| Structure |
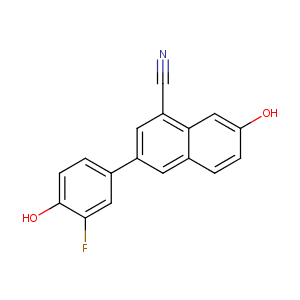 |
Download2D MOL |
||
| Formula |
C17H10FNO2
|
|||
| Canonical SMILES |
C1=CC(=CC2=C(C=C(C=C21)C3=CC(=C(C=C3)O)F)C#N)O
|
|||
| InChI |
1S/C17H10FNO2/c18-16-7-10(2-4-17(16)21)12-5-11-1-3-14(20)8-15(11)13(6-12)9-19/h1-8,20-21H
|
|||
| InChIKey |
NSSOSHDCWCMNDM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 550997-55-2
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Estrogen receptor beta (ESR2) | Target Info | Agonist | [2] |
| KEGG Pathway | Estrogen signaling pathway | |||
| Prolactin signaling pathway | ||||
| Pathway Interaction Database | Plasma membrane estrogen receptor signaling | |||
| Validated nuclear estrogen receptor beta network | ||||
| Validated nuclear estrogen receptor alpha network | ||||
| Reactome | Nuclear Receptor transcription pathway | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Ovarian Infertility Genes | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021229) | |||
| REF 2 | WAY-202196, a selective estrogen receptor-beta agonist, protects against death in experimental septic shock. Crit Care Med. 2006 Aug;34(8):2188-93. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

