Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09NST
|
|||
| Former ID |
DNCL002692
|
|||
| Drug Name |
Afimoxifene
|
|||
| Synonyms |
4-Hydroxytamoxifen; Afimoxifene; (Z)-4-Hydroxytamoxifen; Hydroxytamoxifen; 4-Monohydroxytamoxifen; Tamogel; 68047-06-3; trans-4-Hydroxytamoxifen; Ici 79280; 68392-35-8; 65213-48-1; 4-OH-TAM; (Z)-4-(1-(4-(2-(Dimethylamino)ethoxy)phenyl)-2-phenylbut-1-en-1-yl)phenol; Z-4-hydroxytamoxifen; UNII-95K54647BZ; CHEMBL489; ICI 79,280; TAMOXIFEN, 4-HYDROXY-, (Z)-; BRN 4910749; (E/Z)-4-Hydroxy Tamoxifen; MLS000069742; C26H29NO2; CHEBI:44616; 95K54647BZ; SMR000058939; 4-(1-(4-(2-(Dimethylamino)ethoxy)phenyl)-2-phenylbut-1-enyl)phenol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Breast cancer [ICD-11: 2C60-2C65] | Phase 2 | [1] | |
| Ductal carcinoma [ICD-11: 2E65.2; ICD-10: D05.1; ICD-9: 233] | Phase 2 | [2] | ||
| Company |
ASCEND Therapeutics
|
|||
| Structure |
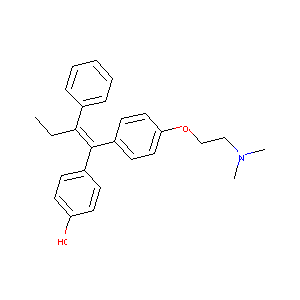 |
Download2D MOL |
||
| Formula |
C26H29NO2
|
|||
| Canonical SMILES |
CCC(=C(C1=CC=C(C=C1)O)C2=CC=C(C=C2)OCCN(C)C)C3=CC=CC=C3
|
|||
| InChI |
1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25-
|
|||
| InChIKey |
TXUZVZSFRXZGTL-QPLCGJKRSA-N
|
|||
| CAS Number |
CAS 68047-06-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7494, 835333, 7889566, 10300358, 11532960, 14719814, 14719815, 14805025, 24895736, 24895814, 24899989, 26757818, 26759601, 36891034, 46393897, 47208207, 48243335, 49688682, 49846252, 50005439, 50065066, 53788270, 53789854, 56422185, 57404921, 57570889, 57654657, 77095723, 85788533, 87219226, 91613892, 93165645, 93167122, 103169566, 104640983, 124799764, 124893066, 126639485, 126671492, 126685161, 134341205, 135021191, 135065359, 135610264, 137156415, 142089319, 144208141, 162226886, 163414603, 163687254
|
|||
| ChEBI ID |
CHEBI:44616
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00272714) Study of Afimoxifene Gel to Treat Cyclic Mastalgia in Premenopausal Women. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 620). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

