Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T89534
(Former ID: TTDS00242)
|
|||||
| Target Name |
Estrogen receptor (ESR)
|
|||||
| Synonyms |
Nuclear receptor subfamily 3 group A member 1; NR3A1; Estradiol receptor; ESR; ER-alpha; ER
Click to Show/Hide
|
|||||
| Gene Name |
ESR1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 15 Target-related Diseases | + | ||||
| 1 | Acne vulgaris [ICD-11: ED80] | |||||
| 2 | Acquired prion disease [ICD-11: 8E01] | |||||
| 3 | Adrenal cancer [ICD-11: 2D11] | |||||
| 4 | Breast cancer [ICD-11: 2C60-2C6Y] | |||||
| 5 | Contraceptive management [ICD-11: QA21] | |||||
| 6 | Dyspareunia [ICD-11: GA12] | |||||
| 7 | Female infertility [ICD-11: GA31] | |||||
| 8 | Low bone mass disorder [ICD-11: FB83] | |||||
| 9 | Male infertility [ICD-11: GB04] | |||||
| 10 | Menopausal disorder [ICD-11: GA30] | |||||
| 11 | Menstrual cycle bleeding disorder [ICD-11: GA20] | |||||
| 12 | Pituitary gland disorder [ICD-11: 5A60-5A61] | |||||
| 13 | Skeletal anomaly [ICD-11: LD24] | |||||
| 14 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 15 | Vaginitis [ICD-11: GA02] | |||||
| Function |
Nuclear hormone receptor. The steroid hormones and their receptors are involved in the regulation of eukaryotic gene expression and affect cellular proliferation and differentiation in target tissues. Ligand-dependent nuclear transactivation involves either direct homodimer binding to a palindromic estrogen response element (ERE) sequence or association with other DNA-binding transcription factors, such as AP-1/c-Jun, c-Fos, ATF-2, Sp1 and Sp3, to mediate ERE-independent signaling. Ligand binding induces a conformational change allowing subsequent or combinatorial association with multiprotein coactivator complexes through LXXLL motifs of their respective components. Mutual transrepression occurs between the estrogen receptor (ER) and NF-kappa-B in a cell-type specific manner. Decreases NF-kappa-B DNA-binding activity and inhibits NF-kappa-B-mediated transcription from the IL6 promoter and displace RELA/p65 and associated coregulators from the promoter. Recruited to the NF-kappa-B response element of the CCL2 and IL8 promoters and can displace CREBBP. Present with NF-kappa-B components RELA/p65 and NFKB1/p50 on ERE sequences. Can also act synergistically with NF-kappa-B to activate transcription involving respective recruitment adjacent response elements; the function involves CREBBP. Can activate the transcriptional activity of TFF1. Also mediates membrane-initiated estrogen signaling involving various kinase cascades. Isoform 3 is involved in activation of NOS3 and endothelial nitric oxide production. Isoforms lacking one or several functional domains are thought to modulate transcriptional activity by competitive ligand or DNA binding and/or heterodimerization with the full-length receptor. Essential for MTA1-mediated transcriptional regulation of BRCA1 and BCAS3. Isoform 3 can bind to ERE and inhibit isoform 1.
Click to Show/Hide
|
|||||
| BioChemical Class |
Nuclear hormone receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MTMTLHTKASGMALLHQIQGNELEPLNRPQLKIPLERPLGEVYLDSSKPAVYNYPEGAAY
EFNAAAAANAQVYGQTGLPYGPGSEAAAFGSNGLGGFPPLNSVSPSPLMLLHPPPQLSPF LQPHGQQVPYYLENEPSGYTVREAGPPAFYRPNSDNRRQGGRERLASTNDKGSMAMESAK ETRYCAVCNDYASGYHYGVWSCEGCKAFFKRSIQGHNDYMCPATNQCTIDKNRRKSCQAC RLRKCYEVGMMKGGIRKDRRGGRMLKHKRQRDDGEGRGEVGSAGDMRAANLWPSPLMIKR SKKNSLALSLTADQMVSALLDAEPPILYSEYDPTRPFSEASMMGLLTNLADRELVHMINW AKRVPGFVDLTLHDQVHLLECAWLEILMIGLVWRSMEHPGKLLFAPNLLLDRNQGKCVEG MVEIFDMLLATSSRFRMMNLQGEEFVCLKSIILLNSGVYTFLSSTLKSLEEKDHIHRVLD KITDTLIHLMAKAGLTLQQQHQRLAQLLLILSHIRHMSNKGMEHLYSMKCKNVVPLYDLL LEMLDAHRLHAPTSRGGASVEETDQSHLATAGSTSSHSLQKYYITGEAEGFPATV Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A00673 ; BADD_A02072 ; BADD_A05322 ; BADD_A08333 | |||||
| HIT2.0 ID | T01QLH | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 37 Approved Drugs | + | ||||
| 1 | ARZOXIFENE | Drug Info | Approved | Breast cancer | [2], [3] | |
| 2 | Bazedoxifene | Drug Info | Approved | Osteopetrosis | [4], [5], [6] | |
| 3 | Cenestin | Drug Info | Approved | Menopause symptom | [5] | |
| 4 | Clomifene | Drug Info | Approved | Female infertility | [7], [8] | |
| 5 | Clomiphene Citrate | Drug Info | Approved | Female infertility | [5] | |
| 6 | Conjugated estrogens a | Drug Info | Approved | Hormone replacement therapy | [5] | |
| 7 | Conjugated estrogens b | Drug Info | Approved | Hormone replacement therapy | [5] | |
| 8 | Cyclofenil | Drug Info | Approved | Infertility | [5] | |
| 9 | Danazol | Drug Info | Approved | Menorrhagia | [9], [10] | |
| 10 | Dienestrol | Drug Info | Approved | Atrophic vaginitis | [5], [11], [12] | |
| 11 | Diethylstilbestrol | Drug Info | Approved | Gonorrheal vaginitis | [5], [13], [14] | |
| 12 | Elacestrant | Drug Info | Approved | Breast cancer | [15] | |
| 13 | Esterified estrogens | Drug Info | Approved | Breast cancer | [5] | |
| 14 | Estradiol | Drug Info | Approved | Breast cancer | [5], [16], [17], [18], [19] | |
| 15 | Estradiol Acetate | Drug Info | Approved | Hormone replacement therapy | [5] | |
| 16 | Estradiol Cypionate | Drug Info | Approved | Hormone replacement therapy | [5] | |
| 17 | Estradiol Valerate | Drug Info | Approved | Hormone replacement therapy | [5] | |
| 18 | Estriol | Drug Info | Approved | Hormone deficiency | [20], [21], [5] | |
| 19 | Estrogen | Drug Info | Approved | Menopause symptom | [5] | |
| 20 | Estrone | Drug Info | Approved | Menopausal and postmenopausal disorder | [22], [23] | |
| 21 | Estropipate | Drug Info | Approved | Hypogonadism | [5] | |
| 22 | Ethinyl Estradiol | Drug Info | Approved | Female hypogonadism | [24], [17] | |
| 23 | Fosfestrol | Drug Info | Approved | Solid tumour/cancer | [5] | |
| 24 | Fulvestrant | Drug Info | Approved | Breast cancer | [25], [17] | |
| 25 | Gestrinone | Drug Info | Approved | Breast cancer | [5] | |
| 26 | Lasofoxifene | Drug Info | Approved | Osteoporosis | [26], [27] | |
| 27 | Levormeloxifene | Drug Info | Approved | Breast cancer | [28] | |
| 28 | Mestranol | Drug Info | Approved | Contraception | [29], [30] | |
| 29 | Mitotane | Drug Info | Approved | Adrenocortical carcinoma | [31], [32] | |
| 30 | Nomegestrol acetate | Drug Info | Approved | Breast cancer | [33], [34], [5] | |
| 31 | Ospemifene | Drug Info | Approved | Dyspareunia | [35], [36] | |
| 32 | Premarin/Trimegestone | Drug Info | Approved | Menopause symptom | [5] | |
| 33 | Promestriene | Drug Info | Approved | Acne vulgaris | [5] | |
| 34 | Quinestrol | Drug Info | Approved | Breast cancer | [37], [38], [5] | |
| 35 | Raloxifene | Drug Info | Approved | Osteoporosis | [5] | |
| 36 | Tamoxifen | Drug Info | Approved | Breast cancer | [39], [40] | |
| 37 | Toremifene | Drug Info | Approved | Breast cancer | [41], [17] | |
| Clinical Trial Drug(s) | [+] 31 Clinical Trial Drugs | + | ||||
| 1 | Acolbifene | Drug Info | Phase 3 | Breast cancer | [42] | |
| 2 | Giredestrant | Drug Info | Phase 3 | Breast cancer | [43] | |
| 3 | Imlunestrant | Drug Info | Phase 3 | Breast cancer | [44] | |
| 4 | NE3107 | Drug Info | Phase 3 | Alzheimer disease | [45] | |
| 5 | NPC-01 | Drug Info | Phase 3 | Dysmenorrhea | [46] | |
| 6 | Premarin/Pravachol | Drug Info | Phase 3 | Hyperlipidaemia | [47] | |
| 7 | Synthetic conjugated estrogen | Drug Info | Phase 3 | Vaginal disease | [48] | |
| 8 | Trimegestone/ethinyl estradiol | Drug Info | Phase 3 | Contraception | [49] | |
| 9 | TZTX-001 | Drug Info | Phase 3 | Endometriosis | [50] | |
| 10 | Afimoxifene | Drug Info | Phase 2 | Breast cancer | [51] | |
| 11 | ARN-810 | Drug Info | Phase 2 | Breast cancer | [52] | |
| 12 | AZD9833 | Drug Info | Phase 2 | ER-positive breast cancer | [53] | |
| 13 | Endoxifen | Drug Info | Phase 2 | Breast cancer | [54], [55] | |
| 14 | Estetrol | Drug Info | Phase 2 | Autoimmune diabetes | [56] | |
| 15 | GTx-758 | Drug Info | Phase 2 | Prostate cancer | [57] | |
| 16 | ICARITIN | Drug Info | Phase 2 | Breast cancer | [58] | |
| 17 | SR 16234 | Drug Info | Phase 2 | Breast cancer | [59] | |
| 18 | H3B-6545 | Drug Info | Phase 1/2 | Breast cancer | [60] | |
| 19 | OP-1250 | Drug Info | Phase 1/2 | Breast cancer | [61] | |
| 20 | ZN-c5 | Drug Info | Phase 1/2 | Breast cancer | [62] | |
| 21 | AC0682 | Drug Info | Phase 1 | Breast cancer | [63] | |
| 22 | ATD transdermal gel | Drug Info | Phase 1 | Contraception | [64] | |
| 23 | AZD9496 | Drug Info | Phase 1 | Breast cancer | [55] | |
| 24 | CC-8490 | Drug Info | Phase 1 | Brain cancer | [65] | |
| 25 | CHF-4227 | Drug Info | Phase 1 | Osteoporosis | [66] | |
| 26 | D-0502 | Drug Info | Phase 1 | Breast cancer | [67] | |
| 27 | G1T-48 | Drug Info | Phase 1 | Breast cancer | [68] | |
| 28 | LY3484356 | Drug Info | Phase 1 | Breast cancer | [69] | |
| 29 | SCO-120 | Drug Info | Phase 1 | Breast cancer | [70] | |
| 30 | TTC-352 | Drug Info | Phase 1 | Breast cancer | [55] | |
| 31 | TTC-352 | Drug Info | Phase 1 | Metastatic melanoma | [71] | |
| Discontinued Drug(s) | [+] 19 Discontinued Drugs | + | ||||
| 1 | BITHIONOL | Drug Info | Withdrawn from market | Trematode infection | [72], [5] | |
| 2 | Chlorotrianisene | Drug Info | Withdrawn from market | Menopause symptom | [73] | |
| 3 | HEXESTROL | Drug Info | Withdrawn from market | Irregularities | [74], [75], [76] | |
| 4 | EM-800 | Drug Info | Discontinued in Phase 3 | Estrogen deficiency | [77] | |
| 5 | Idoxifene | Drug Info | Discontinued in Phase 3 | Breast cancer | [78] | |
| 6 | IoGen | Drug Info | Discontinued in Phase 3 | Pain | [79] | |
| 7 | Miproxifene | Drug Info | Discontinued in Phase 3 | Solid tumour/cancer | [80] | |
| 8 | Droloxifene | Drug Info | Discontinued in Phase 2 | Breast cancer | [81] | |
| 9 | ERA-923 | Drug Info | Discontinued in Phase 2 | Breast cancer | [82] | |
| 10 | NP-50301 | Drug Info | Discontinued in Phase 2 | Eye disorder | [83] | |
| 11 | Panomifene | Drug Info | Discontinued in Phase 2 | Solid tumour/cancer | [84] | |
| 12 | SERM-3339 | Drug Info | Discontinued in Phase 2 | Osteoporosis | [85] | |
| 13 | SR-90067 | Drug Info | Discontinued in Phase 2 | Hormone deficiency | [86] | |
| 14 | HE2100 | Drug Info | Discontinued in Phase 1 | Thrombocytopenia | [87] | |
| 15 | HRT | Drug Info | Discontinued in Phase 1 | Estrogen deficiency | [88] | |
| 16 | MX-4509 | Drug Info | Discontinued in Phase 1 | Neurological disorder | [89] | |
| 17 | ICI-164384 | Drug Info | Terminated | Breast cancer | [91] | |
| 18 | Zindoxifene | Drug Info | Terminated | Breast cancer | [92] | |
| 19 | ZK-119010 | Drug Info | Terminated | Carcinoma | [93] | |
| Preclinical Drug(s) | [+] 6 Preclinical Drugs | + | ||||
| 1 | BN-AA-003-NY | Drug Info | Preclinical | Estrogen deficiency | [90] | |
| 2 | BN-AO-014 | Drug Info | Preclinical | Atrophy | [90] | |
| 3 | BN-CB-045 | Drug Info | Preclinical | Female sexual arousal dysfunction | [90] | |
| 4 | BN-DF-037 | Drug Info | Preclinical | Osteoporosis | [90] | |
| 5 | BN-GU-005-DHP | Drug Info | Preclinical | Arthralgia | [90] | |
| 6 | BN-OD-026 | Drug Info | Preclinical | Solid tumour/cancer | [90] | |
| Mode of Action | [+] 7 Modes of Action | + | ||||
| Inhibitor | [+] 194 Inhibitor drugs | + | ||||
| 1 | ARZOXIFENE | Drug Info | [94] | |||
| 2 | NE3107 | Drug Info | [127] | |||
| 3 | ATD transdermal gel | Drug Info | [121] | |||
| 4 | BITHIONOL | Drug Info | [146] | |||
| 5 | HEXESTROL | Drug Info | [148] | |||
| 6 | ERA-923 | Drug Info | [153], [154] | |||
| 7 | HE2100 | Drug Info | [159] | |||
| 8 | MX-4509 | Drug Info | [161] | |||
| 9 | ICI-164384 | Drug Info | [164] | |||
| 10 | LY-117018 | Drug Info | [165] | |||
| 11 | Tamoxifen methyl iodide | Drug Info | [166] | |||
| 12 | ZK-119010 | Drug Info | [168] | |||
| 13 | 1,2-Bis-(4-hydroxy-phenyl)-3H-inden-5-ol | Drug Info | [169] | |||
| 14 | 1,8-Dichloro-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 15 | 1-Bromo-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 16 | 1-CHLORO-6-(4-HYDROXYPHENYL)-2-NAPHTHOL | Drug Info | [170] | |||
| 17 | 1-Fluoro-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 18 | 17-METHYL-17-ALPHA-DIHYDROEQUILENIN | Drug Info | [171] | |||
| 19 | 2,3-diphenyl-1H-indole | Drug Info | [172] | |||
| 20 | 2,4-Dibenzylamino-6-isopentylpyrimidine | Drug Info | [173] | |||
| 21 | 2,4-diisobutylamino-6-isopentylpyrimidine | Drug Info | [173] | |||
| 22 | 2-(2-Chloro-4-hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 23 | 2-(3-Butoxy-4-hydroxy-phenyl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 24 | 2-(3-Chloro-4-hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 25 | 2-(3-Chloro-4-hydroxy-phenyl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 26 | 2-(3-Fluoro-4-hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 27 | 2-(3-Fluoro-4-hydroxy-phenyl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 28 | 2-(3-hydroxyphenyl)-1,2'-spirobi[1H-indene]-5-ol | Drug Info | [175] | |||
| 29 | 2-(3-hydroxyphenyl)-1,2'-spirobi[1H-indene]-6-ol | Drug Info | [175] | |||
| 30 | 2-(4-Hydroxy-naphthalen-1-yl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 31 | 2-(4-Hydroxy-phenyl)-4-methoxy-quinolin-6-ol | Drug Info | [176] | |||
| 32 | 2-(4-Hydroxy-phenyl)-4-vinyl-quinolin-6-ol | Drug Info | [176] | |||
| 33 | 2-(4-Hydroxy-phenyl)-7-isopropyl-benzooxazol-5-ol | Drug Info | [174] | |||
| 34 | 2-(4-Hydroxy-phenyl)-7-methoxy-benzofuran-5-ol | Drug Info | [177] | |||
| 35 | 2-(4-Hydroxy-phenyl)-7-methoxy-benzooxazol-5-ol | Drug Info | [174] | |||
| 36 | 2-(4-Hydroxy-phenyl)-7-methyl-benzofuran-5-ol | Drug Info | [177] | |||
| 37 | 2-(4-Hydroxy-phenyl)-7-phenyl-benzooxazol-5-ol | Drug Info | [174] | |||
| 38 | 2-(4-Hydroxy-phenyl)-7-propenyl-benzooxazol-5-ol | Drug Info | [174] | |||
| 39 | 2-(4-Hydroxy-phenyl)-7-propyl-benzooxazol-5-ol | Drug Info | [174] | |||
| 40 | 2-(4-Hydroxy-phenyl)-7-vinyl-benzooxazol-5-ol | Drug Info | [174] | |||
| 41 | 2-(4-Hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 42 | 2-(4-Hydroxy-phenyl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 43 | 2-(4-Hydroxy-phenyl)-quinolin-6-ol | Drug Info | [176] | |||
| 44 | 2-(4-HYDROXY-PHENYL)BENZOFURAN-5-OL | Drug Info | [174] | |||
| 45 | 2-(4-hydroxyphenyl)-1,2'-spirobi[1H-indene]-5-ol | Drug Info | [175] | |||
| 46 | 2-(5-Hydroxy-naphthalen-1-yl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 47 | 2-(6-Hydroxy-naphthalen-1-yl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 48 | 2-(6-Hydroxy-naphthalen-1-yl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 49 | 2-(6-Hydroxy-naphthalen-2-yl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 50 | 2-(6-Hydroxy-naphthalen-2-yl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 51 | 2-AMINO-1-METHYL-6-PHENYLIMIDAZO[4,5-B]PYRIDINE | Drug Info | [171] | |||
| 52 | 2-Naphthalen-1-yl-benzooxazol-6-ol | Drug Info | [174] | |||
| 53 | 2-phenyl-1,2'-spirobi[1H-indene]-5'-ol | Drug Info | [175] | |||
| 54 | 3'-Methoxy-4'Hydroxyclomiphene | Drug Info | [178] | |||
| 55 | 3,8-dihydroxy-4-methyl-6H-benzo[c]chromen-6-one | Drug Info | [179] | |||
| 56 | 3,8-dihydroxy-7-methyl-6H-benzo[c]chromen-6-one | Drug Info | [179] | |||
| 57 | 3-(2-Hydroxy-phenyl)-benzo[d]isoxazol-6-ol | Drug Info | [174] | |||
| 58 | 3-(4-Hydroxy-phenyl)-benzo[d]isoxazol-5-ol | Drug Info | [174] | |||
| 59 | 3-(4-Hydroxy-phenyl)-benzo[d]isoxazol-6-ol | Drug Info | [174] | |||
| 60 | 3-(4-Hydroxyphenyl)-7-isobutoxychromen-4-one | Drug Info | [180] | |||
| 61 | 3-(4-Hydroxyphenyl)-7-isopropoxychromen-4-one | Drug Info | [180] | |||
| 62 | 3-(5-Hydroxy-benzooxazol-2-yl)-benzene-1,2-diol | Drug Info | [174] | |||
| 63 | 3-(6-Hydroxy-benzooxazol-2-yl)-benzene-1,2-diol | Drug Info | [174] | |||
| 64 | 3-CHLORO-2-(4-HYDROXYPHENYL)-2H-INDAZOL-5-OL | Drug Info | [171] | |||
| 65 | 3-chloro-4-(4-hydroxyphenyl)salicylaldoxime | Drug Info | [181] | |||
| 66 | 3-ETHYL-2-(4-HYDROXYPHENYL)-2H-INDAZOL-5-OL | Drug Info | [171] | |||
| 67 | 3-hydroxy-8,10-dimethyl-6H-benzo[c]chromen-6-one | Drug Info | [179] | |||
| 68 | 3-[1-ethyl-2-(3-hydroxyphenyl)butyl]phenol | Drug Info | [182] | |||
| 69 | 4',5,7-trihydroxy-6,8-dimethylisoflavone | Drug Info | [183] | |||
| 70 | 4,10-dimethyl-6H-benzo[c]chromene-3,8-diol | Drug Info | [179] | |||
| 71 | 4,6,10-trimethyl-6H-benzo[c]chromene-3,8-diol | Drug Info | [179] | |||
| 72 | 4,6,6,7-tetramethyl-6H-benzo[c]chromene-3,8-diol | Drug Info | [179] | |||
| 73 | 4,6,7,10-tetramethyl-6H-benzo[c]chromene-3,8-diol | Drug Info | [179] | |||
| 74 | 4,6,7-trimethyl-6H-benzo[c]chromene-3,8-diol | Drug Info | [179] | |||
| 75 | 4,7-dimethyl-6H-benzo[c]chromene-3,8-diol | Drug Info | [179] | |||
| 76 | 4-(1,2-Diphenyl-but-1-enyl)-phenol | Drug Info | [184] | |||
| 77 | 4-(1-benzyl-7-chloro-1H-indazol-3-yl)phenol | Drug Info | [185] | |||
| 78 | 4-(1-butyl-7-chloro-1H-indazol-3-yl)phenol | Drug Info | [185] | |||
| 79 | 4-(1-cyclopentyl-7-fluoro-1H-indazol-3-yl)phenol | Drug Info | [185] | |||
| 80 | 4-(2-phenyl-1H-benzo[d]imidazol-1-yl)phenol | Drug Info | [172] | |||
| 81 | 4-(2-phenyl-1H-indol-3-yl)phenol | Drug Info | [172] | |||
| 82 | 4-(3-(4-hydroxyphenyl)-1H-indol-2-yl)phenol | Drug Info | [172] | |||
| 83 | 4-(3-phenyl-1H-indol-2-yl)phenol | Drug Info | [172] | |||
| 84 | 4-(5-Hydroxy-benzooxazol-2-yl)-benzene-1,3-diol | Drug Info | [174] | |||
| 85 | 4-(6-HYDROXY-1H-INDAZOL-3-YL)BENZENE-1,3-DIOL | Drug Info | [171] | |||
| 86 | 4-(6-Hydroxy-benzooxazol-2-yl)-benzene-1,2-diol | Drug Info | [174] | |||
| 87 | 4-(6-Hydroxy-benzooxazol-2-yl)-benzene-1,3-diol | Drug Info | [174] | |||
| 88 | 4-(7-chloro-1-cyclohexyl-1H-indazol-3-yl)phenol | Drug Info | [185] | |||
| 89 | 4-(7-chloro-1-cyclopentyl-1H-indazol-3-yl)phenol | Drug Info | [185] | |||
| 90 | 4-(7-chloro-1-propyl-1H-indazol-3-yl)phenol | Drug Info | [185] | |||
| 91 | 4-(7-methyl-1-propyl-1H-indazol-3-yl)phenol | Drug Info | [185] | |||
| 92 | 4-Benzo[d]isoxazol-3-yl-benzene-1,3-diol | Drug Info | [174] | |||
| 93 | 4-benzyl-2,6-diisobutylamino-pyrimidine | Drug Info | [173] | |||
| 94 | 4-Bromo-2-(4-hydroxy-phenyl)-quinolin-6-ol | Drug Info | [176] | |||
| 95 | 4-Chloro-2-(4-hydroxy-phenyl)-quinolin-6-ol | Drug Info | [176] | |||
| 96 | 4-Ethyl-2-(4-hydroxy-phenyl)-quinolin-6-ol | Drug Info | [176] | |||
| 97 | 4-Ethynyl-2-(4-hydroxy-phenyl)-quinolin-6-ol | Drug Info | [176] | |||
| 98 | 4-hydroxy-N,N-diphenylbenzenesulfonamide | Drug Info | [186] | |||
| 99 | 4-hydroxy-N-isopropyl-N-phenylbenzenesulfonamide | Drug Info | [186] | |||
| 100 | 4-hydroxy-N-neopentyl-N-phenylbenzenesulfonamide | Drug Info | [186] | |||
| 101 | 4-hydroxy-N-phenyl-N-propylbenzenesulfonamide | Drug Info | [186] | |||
| 102 | 4-Naphthalen-2-yl-phenol | Drug Info | [170] | |||
| 103 | 4-[1,2-bis(4-hydroxyphenyl)but-1-enyl]phenol | Drug Info | [187] | |||
| 104 | 4-[1,2-bis(4-hydroxyphenyl)hex-1-enyl]phenol | Drug Info | [187] | |||
| 105 | 4-[1,2-bis(4-hydroxyphenyl)pent-1-enyl]phenol | Drug Info | [187] | |||
| 106 | 4-[1,2-bis(4-hydroxyphenyl)vinyl]phenol | Drug Info | [187] | |||
| 107 | 4-[1-(4-hydroxyphenyl)-2-phenylbut-1-enyl]phenol | Drug Info | [184] | |||
| 108 | 4-[1-(4-hydroxyphenyl)-2-phenylhex-1-enyl]phenol | Drug Info | [184] | |||
| 109 | 4-[1-(4-hydroxyphenyl)-2-phenylpent-1-enyl]phenol | Drug Info | [184] | |||
| 110 | 4-[1-(4-hydroxyphenyl)-2-phenylprop-1-enyl]phenol | Drug Info | [184] | |||
| 111 | 4-[1-(4-hydroxyphenyl)-2-phenylvinyl]phenol | Drug Info | [184] | |||
| 112 | 4-[2,2-bis(4-hydroxyphenyl)-1-methylvinyl]phenol | Drug Info | [187] | |||
| 113 | 5-Bromo-2-(4-hydroxy-phenyl)-quinolin-6-ol | Drug Info | [176] | |||
| 114 | 5-Chloro-2-(4-hydroxy-phenyl)-benzooxazol-6-ol | Drug Info | [174] | |||
| 115 | 5-Chloro-2-(4-hydroxy-phenyl)-quinolin-6-ol | Drug Info | [176] | |||
| 116 | 5-hydroxy-2-phenylisoindoline-1,3-dione | Drug Info | [188] | |||
| 117 | 6-(2,5-Difluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 118 | 6-(2,6-Difluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 119 | 6-(2-Chloro-4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 120 | 6-(2-Fluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 121 | 6-(3,5-Difluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 122 | 6-(3-Chloro-4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 123 | 6-(3-Fluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 124 | 6-(3-Hydroxy-phenyl)-naphthalen-1-ol | Drug Info | [170] | |||
| 125 | 6-(3-Hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 126 | 6-(4-Hydroxy-2-methoxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 127 | 6-(4-Hydroxy-2-methyl-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 128 | 6-(4-Hydroxy-phenyl)-1-methoxy-naphthalen-2-ol | Drug Info | [170] | |||
| 129 | 6-(4-Hydroxy-phenyl)-1-methyl-naphthalen-2-ol | Drug Info | [170] | |||
| 130 | 6-(4-Hydroxy-phenyl)-1-nitro-naphthalen-2-ol | Drug Info | [170] | |||
| 131 | 6-(4-Hydroxy-phenyl)-1-phenyl-naphthalen-2-ol | Drug Info | [170] | |||
| 132 | 6-(4-Hydroxy-phenyl)-naphthalen-1-ol | Drug Info | [170] | |||
| 133 | 6-(4-Hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [176] | |||
| 134 | 6-butyl-2,4-dipropylaminopyrimidine | Drug Info | [173] | |||
| 135 | 6-Chloro-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 136 | 6-ethyl-2,4-diisobutylaminopyrimidine | Drug Info | [173] | |||
| 137 | 6-ethyl-4,7-dimethyl-6H-benzo[c]chromene-3,8-diol | Drug Info | [179] | |||
| 138 | 6-Phenyl-naphthalen-2-ol | Drug Info | [170] | |||
| 139 | 7-(3-Hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 140 | 7-(4-Hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 141 | 7-Allyl-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 142 | 7-Bromo-2-(4-hydroxy-phenyl)-benzofuran-5-ol | Drug Info | [177] | |||
| 143 | 7-Butyl-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 144 | 7-Chloro-2-(4-hydroxy-phenyl)-benzofuran-5-ol | Drug Info | [177] | |||
| 145 | 7-Cyclopentyloxy-3-(4-hydroxyphenyl)chromen-4-one | Drug Info | [180] | |||
| 146 | 7-Ethyl-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 147 | 7-Ethynyl-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Drug Info | [174] | |||
| 148 | 7-Phenyl-naphthalen-2-ol | Drug Info | [170] | |||
| 149 | 8-(2,2-dimethylpropyl)naringenin | Drug Info | [189] | |||
| 150 | 8-(2-methylpropyl)naringenin | Drug Info | [189] | |||
| 151 | 8-(3-methylbutyl)naringenin | Drug Info | [189] | |||
| 152 | 8-benzylnaringenin | Drug Info | [189] | |||
| 153 | 8-Chloro-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 154 | 8-Fluoro-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Drug Info | [170] | |||
| 155 | 8-methylnaringenin | Drug Info | [189] | |||
| 156 | 8-n-heptylnaringenin | Drug Info | [189] | |||
| 157 | 8-n-nonylnaringenin | Drug Info | [189] | |||
| 158 | 8-n-pentylnaringenin | Drug Info | [189] | |||
| 159 | 8-n-propylnaringenin | Drug Info | [189] | |||
| 160 | 8-n-undecylnaringenin | Drug Info | [189] | |||
| 161 | BROUSSONIN A | Drug Info | [190] | |||
| 162 | Carboron Cluster with phenol | Drug Info | [191] | |||
| 163 | COUMESTROL | Drug Info | [192] | |||
| 164 | CP-394531 | Drug Info | [193] | |||
| 165 | CP-409069 | Drug Info | [193] | |||
| 166 | daidzein | Drug Info | [183] | |||
| 167 | DIHYDRORALOXIFENE | Drug Info | [194] | |||
| 168 | Doxorubicin-Formaldehyde Conjugate | Drug Info | [195] | |||
| 169 | EFFUSOL | Drug Info | [179] | |||
| 170 | Geldanamycin-estradiol hybrid | Drug Info | [197] | |||
| 171 | GSK-5182 | Drug Info | [198] | |||
| 172 | JNJ-17148066 | Drug Info | [200] | |||
| 173 | JNJ-19398990 | Drug Info | [200] | |||
| 174 | JNJ-26529126 | Drug Info | [200] | |||
| 175 | JNJ-26529152 | Drug Info | [200] | |||
| 176 | LTERHKILHRLLQEGSPSD | Drug Info | [201] | |||
| 177 | MPrP | Drug Info | [203] | |||
| 178 | N,N,N-Triisobutyl-pyrimidine-2,4,6-triamine | Drug Info | [173] | |||
| 179 | N-allyl-4-hydroxy-N-phenylbenzenesulfonamide | Drug Info | [186] | |||
| 180 | N-benzyl-4-hydroxy-N-phenylbenzenesulfonamide | Drug Info | [186] | |||
| 181 | N-butyl-4-hydroxy-N-phenylbenzenesulfonamide | Drug Info | [186] | |||
| 182 | N-cyclohexyl-4-hydroxy-N-phenylbenzenesulfonamide | Drug Info | [186] | |||
| 183 | N-ethyl-4-hydroxy-N-phenylbenzenesulfonamide | Drug Info | [186] | |||
| 184 | Nafoxidine | Drug Info | [204] | |||
| 185 | SNG-163 | Drug Info | [121] | |||
| 186 | SNG-8033 | Drug Info | [121] | |||
| 187 | SOPHORAFLAVANONE B | Drug Info | [189] | |||
| 188 | Tamoxifen butyl bromide | Drug Info | [166] | |||
| 189 | Tamoxifen ethyl bromide | Drug Info | [166] | |||
| 190 | Tamoxifen isopropyl bromide | Drug Info | [166] | |||
| 191 | WAY-169916 | Drug Info | [185] | |||
| 192 | WAY200070 | Drug Info | [174] | |||
| 193 | ZK-164015 | Drug Info | [208] | |||
| 194 | [1,1':2',1'']Terphenyl-4'-carbaldehyde oxime | Drug Info | [209] | |||
| Modulator | [+] 58 Modulator drugs | + | ||||
| 1 | Bazedoxifene | Drug Info | [5], [95] | |||
| 2 | Clomifene | Drug Info | [98] | |||
| 3 | Clomiphene Citrate | Drug Info | [99] | |||
| 4 | Conjugated estrogens a | Drug Info | [17] | |||
| 5 | Conjugated estrogens b | Drug Info | [100], [17] | |||
| 6 | Cyclofenil | Drug Info | [101], [5] | |||
| 7 | Elacestrant | Drug Info | [105] | |||
| 8 | Esterified estrogens | Drug Info | [106], [5] | |||
| 9 | Estradiol Acetate | Drug Info | [99] | |||
| 10 | Estradiol Cypionate | Drug Info | [99] | |||
| 11 | Estradiol Valerate | Drug Info | [99] | |||
| 12 | Estropipate | Drug Info | [109], [5] | |||
| 13 | Fosfestrol | Drug Info | [111], [112] | |||
| 14 | Gestrinone | Drug Info | [17] | |||
| 15 | Lasofoxifene | Drug Info | [27] | |||
| 16 | Levormeloxifene | Drug Info | [113], [5] | |||
| 17 | Nomegestrol acetate | Drug Info | [5], [33], [34] | |||
| 18 | Ospemifene | Drug Info | [117] | |||
| 19 | Premarin/Trimegestone | Drug Info | [118], [5] | |||
| 20 | Promestriene | Drug Info | [119], [5] | |||
| 21 | Quinestrol | Drug Info | [120] | |||
| 22 | Raloxifene | Drug Info | [121] | |||
| 23 | Toremifene | Drug Info | [124] | |||
| 24 | Acolbifene | Drug Info | [125] | |||
| 25 | NPC-01 | Drug Info | [46] | |||
| 26 | Premarin/Pravachol | Drug Info | [47] | |||
| 27 | Synthetic conjugated estrogen | Drug Info | [128], [5] | |||
| 28 | Trimegestone/ethinyl estradiol | Drug Info | [129] | |||
| 29 | TZTX-001 | Drug Info | [130] | |||
| 30 | Afimoxifene | Drug Info | [121] | |||
| 31 | ARN-810 | Drug Info | [131] | |||
| 32 | Endoxifen | Drug Info | [121] | |||
| 33 | Estetrol | Drug Info | [133] | |||
| 34 | GTx-758 | Drug Info | [134] | |||
| 35 | ICARITIN | Drug Info | [135] | |||
| 36 | SR 16234 | Drug Info | [136] | |||
| 37 | CC-8490 | Drug Info | [140] | |||
| 38 | CHF-4227 | Drug Info | [141] | |||
| 39 | Idoxifene | Drug Info | [150] | |||
| 40 | IoGen | Drug Info | [121] | |||
| 41 | Miproxifene | Drug Info | [151] | |||
| 42 | Droloxifene | Drug Info | [152] | |||
| 43 | NP-50301 | Drug Info | [155] | |||
| 44 | Panomifene | Drug Info | [156] | |||
| 45 | SERM-3339 | Drug Info | [157] | |||
| 46 | BN-AA-003-NY | Drug Info | [162] | |||
| 47 | BN-AO-014 | Drug Info | [162] | |||
| 48 | BN-CB-045 | Drug Info | [162] | |||
| 49 | BN-DF-037 | Drug Info | [162] | |||
| 50 | BN-GU-005-DHP | Drug Info | [162] | |||
| 51 | BN-OD-026 | Drug Info | [163] | |||
| 52 | Zindoxifene | Drug Info | [167] | |||
| 53 | Estriol E3 | Drug Info | [196] | |||
| 54 | Org-37663 | Drug Info | [205] | |||
| 55 | RG6046 | Drug Info | [5] | |||
| 56 | SERMs | Drug Info | [121] | |||
| 57 | STX | Drug Info | [121] | |||
| 58 | TSERaM | Drug Info | [121] | |||
| Agonist | [+] 15 Agonist drugs | + | ||||
| 1 | Cenestin | Drug Info | [5], [96], [97] | |||
| 2 | Dienestrol | Drug Info | [103] | |||
| 3 | Diethylstilbestrol | Drug Info | [104] | |||
| 4 | Estradiol | Drug Info | [107] | |||
| 5 | Estriol | Drug Info | [107] | |||
| 6 | Estrogen | Drug Info | [108] | |||
| 7 | Estrone | Drug Info | [107] | |||
| 8 | Ethinyl Estradiol | Drug Info | [110], [107] | |||
| 9 | Mestranol | Drug Info | [114] | |||
| 10 | TTC-352 | Drug Info | [55] | |||
| 11 | TTC-352 | Drug Info | [145] | |||
| 12 | SR-90067 | Drug Info | [158] | |||
| 13 | HRT | Drug Info | [160] | |||
| 14 | propylpyrazoletriol | Drug Info | [206] | |||
| 15 | R,R-THC | Drug Info | [207] | |||
| Antagonist | [+] 11 Antagonist drugs | + | ||||
| 1 | Danazol | Drug Info | [102] | |||
| 2 | Fulvestrant | Drug Info | [1] | |||
| 3 | Tamoxifen | Drug Info | [122], [123] | |||
| 4 | H3B-6545 | Drug Info | [55] | |||
| 5 | OP-1250 | Drug Info | [137] | |||
| 6 | AZD9496 | Drug Info | [55] | |||
| 7 | EM-800 | Drug Info | [149] | |||
| 8 | GW7604 | Drug Info | [199] | |||
| 9 | methyl-piperidino-pyrazole | Drug Info | [202] | |||
| 10 | SNG-8006 | Drug Info | [121] | |||
| 11 | Trans-hydroxytamoxifen | Drug Info | [202] | |||
| Binder | [+] 2 Binder drugs | + | ||||
| 1 | Mitotane | Drug Info | [115], [116] | |||
| 2 | Chlorotrianisene | Drug Info | [147] | |||
| Degrader | [+] 1 Degrader drugs | + | ||||
| 1 | Giredestrant | Drug Info | [126] | |||
| Degrader | [+] 8 Degrader drugs | + | ||||
| 1 | Imlunestrant | Drug Info | [44] | |||
| 2 | AZD9833 | Drug Info | [132] | |||
| 3 | ZN-c5 | Drug Info | [138] | |||
| 4 | AC0682 | Drug Info | [139] | |||
| 5 | D-0502 | Drug Info | [142] | |||
| 6 | G1T-48 | Drug Info | [143] | |||
| 7 | LY3484356 | Drug Info | [69] | |||
| 8 | SCO-120 | Drug Info | [144] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Clomifene | Ligand Info | |||||
| Structure Description | Estrogen Receptor Alpha Ligand Binding Domain in Complex with the Selective Estrogen Receptor Modulator Clomiphene | PDB:6VPF | ||||
| Method | X-ray diffraction | Resolution | 1.60 Å | Mutation | No | [210] |
| PDB Sequence |
LALSLTADQM
315 VSALLDAEPP325 ILYSEYDPTR335 PFSEASMMGL345 LTNLADRELV355 HMINWAKRVP 365 GFVDLTLHDQ375 VHLLESAWLE385 ILMIGLVWRS395 MEHPGKLLFA405 PNLLLDRNQG 415 KSVEGMVEIF425 DMLLATSSRF435 RMMNLQGEEF445 VCLKSIILLN455 SGVYTFLSST 465 LKSLEEKDHI475 HRVLDKITDT485 LIHLMAKAGL495 TLQQQHQRLA505 QLLLILSHIR 515 HMSNKGMEHL525 YSMKKNVVPS536 YDLLLEMLDA546 HRL
|
|||||
|
|
MET343
3.552
LEU346
3.642
THR347
3.526
LEU349
3.810
ALA350
3.378
ASP351
3.002
GLU353
3.840
LEU354
3.981
TRP383
3.286
LEU384
3.890
LEU387
3.716
MET388
4.371
LEU391
4.017
|
|||||
| Ligand Name: Bazedoxifene | Ligand Info | |||||
| Structure Description | Bazedoxifene in Complex with Y537S Estrogen Receptor Alpha Ligand Binding Domain | PDB:6PSJ | ||||
| Method | X-ray diffraction | Resolution | 1.80 Å | Mutation | Yes | [210] |
| PDB Sequence |
ALSLTADQMV
316 SALLDAEPPI326 LYSESMMGLL346 TNLADRELVH356 MINWAKRVPG366 FVDLTLHDQV 376 HLLESAWLEI386 LMIGLVWRSM396 EHPGKLLFAP406 NLLLDRNQGK416 SVEGMVEIFD 426 MLLATSSRFR436 MMNLQGEEFV446 CLKSIILLNS456 GVYTFLSSTL466 KSLEEKDHIH 476 RVLDKITDTL486 IHLMAKAGLT496 LQQQHQRLAQ506 LLLILSHIRH516 MSNKGMEHLY 526 SMKNVVPLSD538 LLLEMLDAHR548 LH
|
|||||
|
|
MET343
4.028
LEU346
3.626
THR347
3.773
LEU349
3.774
ALA350
3.815
ASP351
2.637
GLU353
2.295
LEU354
3.803
TRP383
3.693
LEU384
4.113
LEU387
3.815
MET388
3.756
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|

| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Estrogen signaling pathway | hsa04915 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
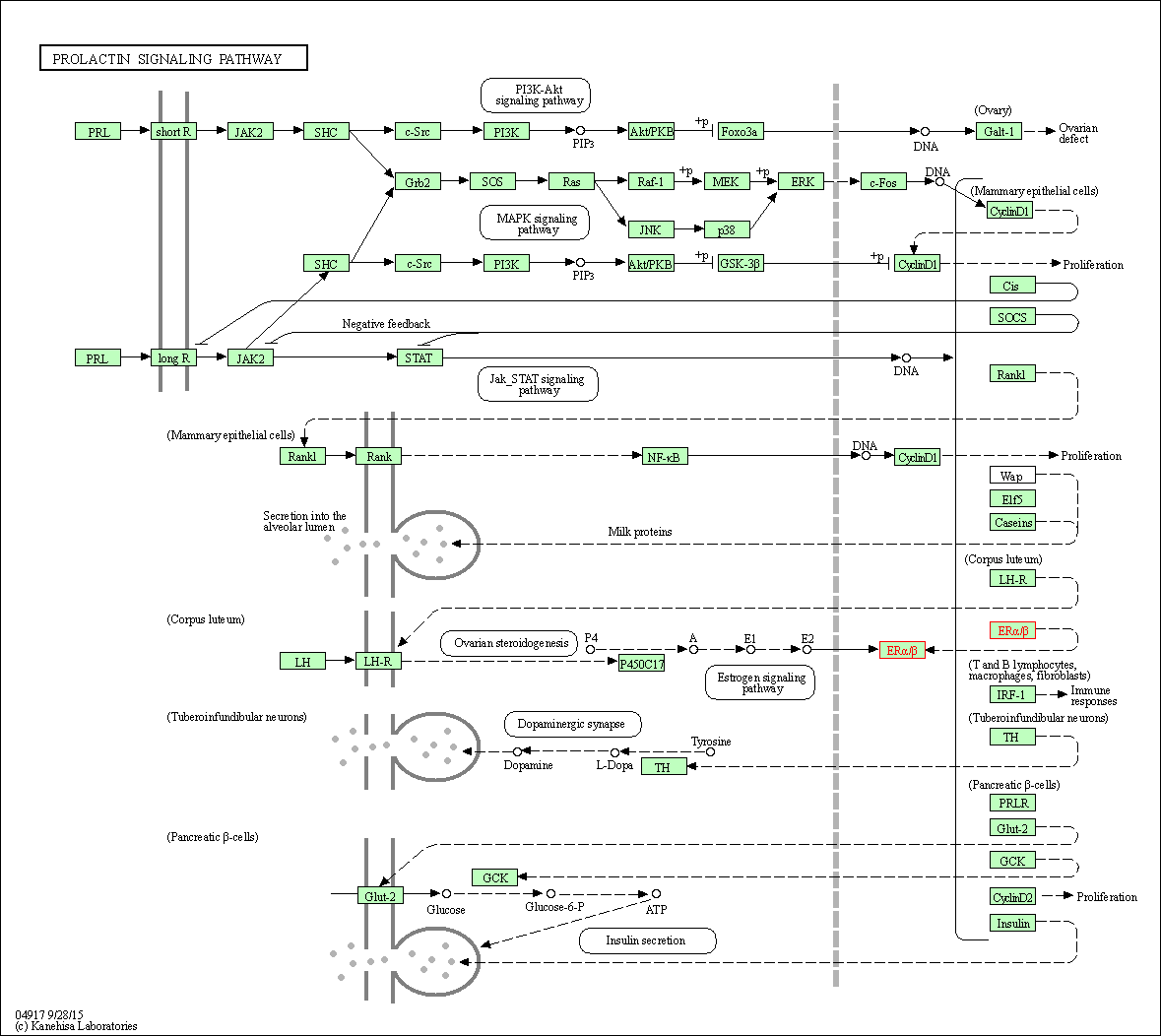
| Prolactin signaling pathway | hsa04917 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
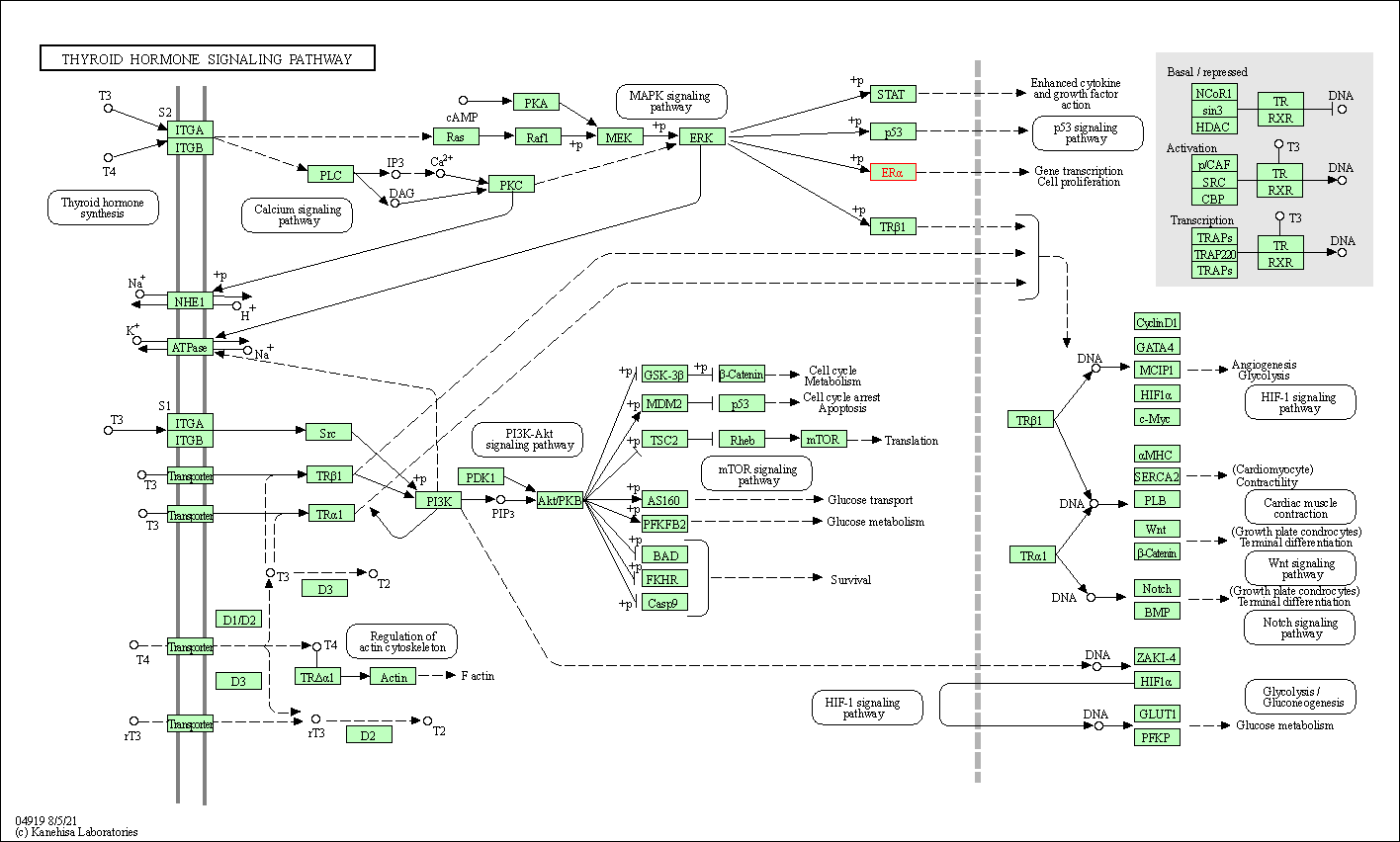
| Thyroid hormone signaling pathway | hsa04919 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
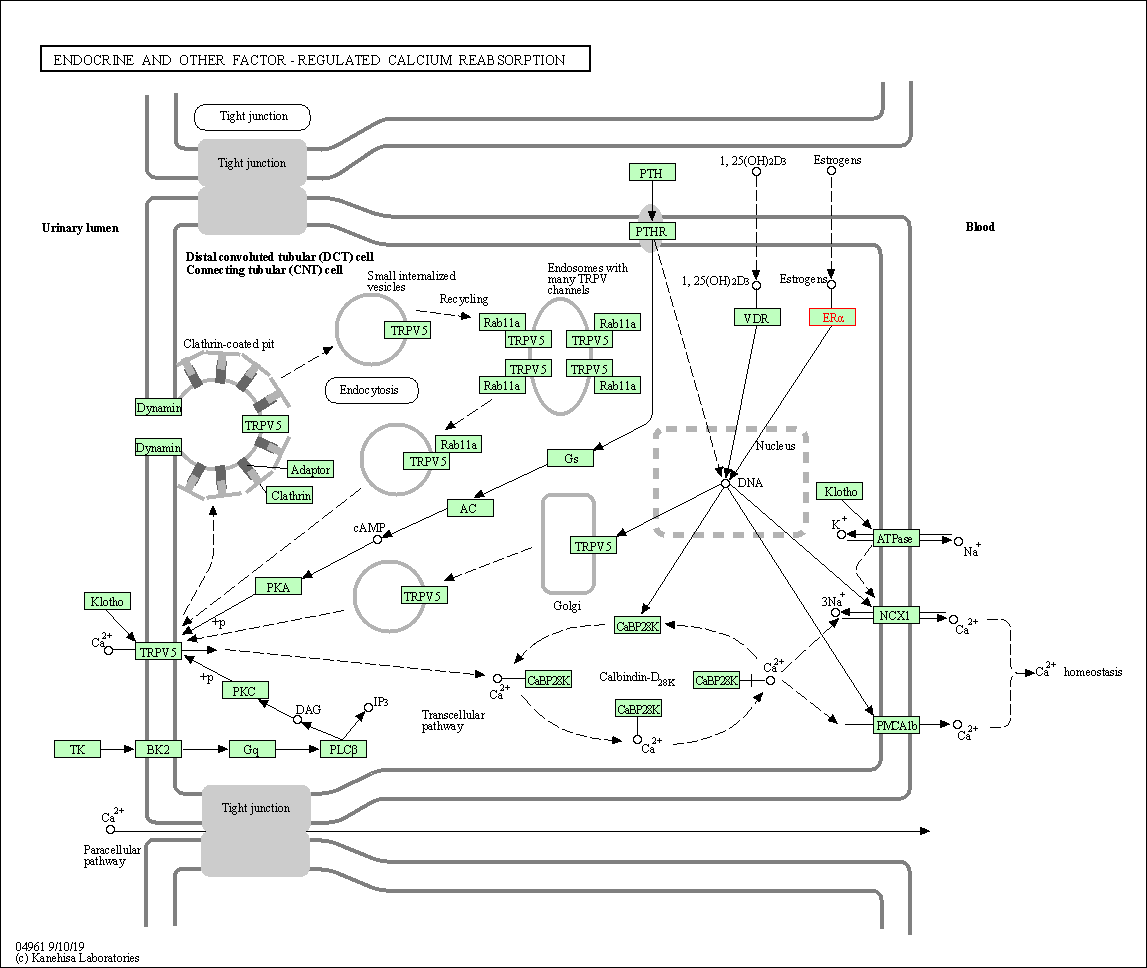
| Endocrine and other factor-regulated calcium reabsorption | hsa04961 | Affiliated Target |

|
| Class: Organismal Systems => Excretory system | Pathway Hierarchy | ||
| Degree | 102 | Degree centrality | 1.10E-02 | Betweenness centrality | 1.69E-02 |
|---|---|---|---|---|---|
| Closeness centrality | 2.83E-01 | Radiality | 1.48E+01 | Clustering coefficient | 8.13E-02 |
| Neighborhood connectivity | 4.43E+01 | Topological coefficient | 2.50E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Drug Resistance Mutation (DRM) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Beta3-tubulin is induced by estradiol in human breast carcinoma cells through an estrogen-receptor dependent pathway. Cell Motil Cytoskeleton. 2009 Jul;66(7):378-88. | |||||
| REF 2 | Clinical pipeline report, company report or official report of Lilly. | |||||
| REF 3 | ClinicalTrials.gov (NCT00190697) A Study of LY353381 (Arzoxifene) for Patients Who Benefitted From This Drug in Other Oncology Trials and Wished to Continue Treatment. U.S. National Institutes of Health. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7355). | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 6 | ClinicalTrials.gov (NCT02090400) Switching From Oral Bisphosphonates to Bazedoxifene to Evaluate Effects on Bone Mineral Density in Postmenopausal Women. U.S. National Institutes of Health. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7619). | |||||
| REF 8 | Emerging drugs for hypogonadism. Expert Opin Emerg Drugs. 2006 Nov;11(4):685-707. | |||||
| REF 9 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6942). | |||||
| REF 10 | Emerging drugs for idiopathic thrombocytopenic purpura in adults. Expert Opin Emerg Drugs. 2008 Jun;13(2):237-54. | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7160). | |||||
| REF 12 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 006110. | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2801). | |||||
| REF 14 | Drug information of Diethylstilbestrol, 2008. eduDrugs. | |||||
| REF 15 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 217639. | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1013). | |||||
| REF 17 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 18 | Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol Endocrinol. 1988 Dec;2(12):1157-62. | |||||
| REF 19 | Estradiol regulates estrogen receptor mRNA stability. J Steroid Biochem Mol Biol. 1998 Aug;66(3):113-20. | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2821). | |||||
| REF 21 | Drug information of Estriol, 2008. eduDrugs. | |||||
| REF 22 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2818). | |||||
| REF 23 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 085239. | |||||
| REF 24 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7071). | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1015). | |||||
| REF 26 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7542). | |||||
| REF 27 | Pfizer. Product Development Pipeline. March 31 2009. | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009670) | |||||
| REF 29 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7087). | |||||
| REF 30 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 010976. | |||||
| REF 31 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6957). | |||||
| REF 32 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 016885. | |||||
| REF 33 | Regulation of rat uterine steroid receptors by nomegestrol acetate, a new 19-nor-progesterone derivative. J Pharmacol Exp Ther. 1989 Feb;248(2):758-61. | |||||
| REF 34 | An overview of nomegestrol acetate selective receptor binding and lack of estrogenic action on hormone-dependent cancer cells. J Steroid Biochem Mol Biol. 2003 Nov;87(2-3):111-22. | |||||
| REF 35 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7349). | |||||
| REF 36 | Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651. | |||||
| REF 37 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7097). | |||||
| REF 38 | Drug information of Quinestrol, 2008. eduDrugs. | |||||
| REF 39 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1016). | |||||
| REF 40 | Rational management of endocrine resistance in breast cancer: a comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer. 2008 Nov 1;113(9):2385-97. | |||||
| REF 41 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4325). | |||||
| REF 42 | ClinicalTrials.gov (NCT01452373) Dehydroepiandrosterone (DHEA) + Acolbifene Against Vasomotor Symptoms (Hot Flushes) in Postmenopausal Women. U.S. National Institutes of Health. | |||||
| REF 43 | ClinicalTrials.gov (NCT04961996) A Phase III, Randomized, Open-Label, Multicenter Study Evaluating the Efficacy and Safety of Adjuvant Giredestrant Compared With Physician's Choice of Adjuvant Endocrine Monotherapy in Patients With Estrogen Receptor-Positive, HER2-Negative Early Breast Cancer. U.S.National Institutes of Health. | |||||
| REF 44 | ClinicalTrials.gov (NCT04975308) EMBER-3: A Phase 3, Randomized, Open-Label Study of Imlunestrant, Investigator's Choice of Endocrine Therapy, and Imlunestrant Plus Abemaciclib in Patients With Estrogen Receptor Positive, HER2 Negative Locally Advanced or Metastatic Breast Cancer Previously Treated With Endocrine Therapy. U.S.National Institutes of Health. | |||||
| REF 45 | ClinicalTrials.gov (NCT04669028) A Phase 3, Double Blind, Randomized, Placebo Controlled, Parallel Group, Multicenter Study of NE3107 in Subjects Who Have Mild to Moderate Probable Alzheimer's Disease. U.S.National Institutes of Health. | |||||
| REF 46 | ClinicalTrials.gov (NCT01246791) Pharmacokinetics of NPC-01 After Single Oral Administration in Healthy Female Volunteers. U.S. National Institutes of Health. | |||||
| REF 47 | Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat Rev Drug Discov. 2003 Jul;2(7):517-26. | |||||
| REF 48 | ClinicalTrials.gov (NCT00361569) A Clinical Trial to Evaluate the Safety and Efficacy of DR-2041(Synthetic Conjugated Estrogens, A) for Treatment of Vulvovaginal Atrophy. U.S. National Institutes of Health. | |||||
| REF 49 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800016303) | |||||
| REF 50 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800038089) | |||||
| REF 51 | ClinicalTrials.gov (NCT00272714) Study of Afimoxifene Gel to Treat Cyclic Mastalgia in Premenopausal Women. U.S. National Institutes of Health. | |||||
| REF 52 | ClinicalTrials.gov (NCT01823835) A Study of ARN-810 (GDC-0810) in Postmenopausal Women With Locally Advanced or Metastatic Estrogen Receptor Positive Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 53 | ClinicalTrials.gov (NCT04214288) A Comparative Study of AZD9833 Versus Fulvestrant in Women With Advanced ER-Positive HER2-Negative Breast Cancer (SERENA-2). U.S. National Institutes of Health. | |||||
| REF 54 | ClinicalTrials.gov (NCT02311933) Tamoxifen Citrate or Z-Endoxifen Hydrochloride in Treating Patients With Locally Advanced or Metastatic, Estrogen Receptor-Positive, HER2-Negative Breast Cancer. U.S.National Institutes of Health. | |||||
| REF 55 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 56 | ClinicalTrials.gov (NCT00563472) Feasibility Study Into the Contraceptive Effect of Estetrol. U.S. National Institutes of Health. | |||||
| REF 57 | ClinicalTrials.gov (NCT01326312) Effect of GTx-758 on Total and Free Testosterone Levels in Men With Prostate Cancer. U.S. National Institutes of Health. | |||||
| REF 58 | ClinicalTrials.gov (NCT01972672) The Phase II Study of Icaritin in Patients With Advanced Hepatocellular Carcinoma. U.S. National Institutes of Health. | |||||
| REF 59 | Randomized double-blind phase 2 trial of 3 doses of TAS-108 in patients with advanced or metastatic postmenopausal breast cancer. Cancer. 2012 Jul 1;118(13):3244-53. | |||||
| REF 60 | ClinicalTrials.gov (NCT03250676) Trial of H3B-6545, in Women With Locally Advanced or Metastatic Estrogen Receptor-positive, HER2 Negative Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 61 | ClinicalTrials.gov (NCT04505826) A Dose Escalation/Expansion Study of Oral OP-1250 in Subjects With Advanced and/or Metastatic HR+, HER2- Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 62 | ClinicalTrials.gov (NCT03560531) A Study of ZN-c5 in Subjects With Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 63 | ClinicalTrials.gov (NCT05489679) A Phase I Clinical Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Preliminary Anti-Tumor Activity of AC682 In Chinese Patients With ER+/HER2- Locally Advanced or Metastatic Breast Cancer. U.S.National Institutes of Health. | |||||
| REF 64 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024900) | |||||
| REF 65 | ClinicalTrials.gov (NCT00074646) Phase I Trial of CC-8490 for the Treatment of Subjects With Recurrent/Refractory High-Grade Gliomas. U.S. National Institutes of Health. | |||||
| REF 66 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800016760) | |||||
| REF 67 | ClinicalTrials.gov (NCT03471663) A First-in-Human Study of D-0502 Alone and in Combination With Palbociclib in Women With Advanced or Metastatic ER-Positive and HER2-Negative Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 68 | ClinicalTrials.gov (NCT03455270) G1T48, an Oral SERD, Alone and in Combination With Palbociclib in ER-Positive, HER2-Negative Advanced Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 69 | ClinicalTrials.gov (NCT04647487) A Study of LY3484356 in Women With Breast Cancer Before Having Surgery (EMBER-2). U.S. National Institutes of Health. | |||||
| REF 70 | ClinicalTrials.gov (NCT04242953) Study to Determine Safety, Tolerability and Pharmacokinetics, of SCO-120 in Healthy Male and Post-menopausal Female Subjects. U.S. National Institutes of Health. | |||||
| REF 71 | ClinicalTrials.gov (NCT03201913) Study of TTC-352 in Patients With Metastatic Breast Cancer Progressing on Endocrine Therapy. U.S. National Institutes of Health. | |||||
| REF 72 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2338). | |||||
| REF 73 | FDA Approved Drug Products from FDA Official Website. 2008. Application Number: (ANDA) 017675. | |||||
| REF 74 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2823). | |||||
| REF 75 | Carcinogenicity and metabolic activation of hexestrol. Chem Biol Interact. 1985 Oct;55(1-2):157-76. | |||||
| REF 76 | Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997 Mar;138(3):863-70. | |||||
| REF 77 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009862) | |||||
| REF 78 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003448) | |||||
| REF 79 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011554) | |||||
| REF 80 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000796) | |||||
| REF 81 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000847) | |||||
| REF 82 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011640) | |||||
| REF 83 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022636) | |||||
| REF 84 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002425) | |||||
| REF 85 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013842) | |||||
| REF 86 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010354) | |||||
| REF 87 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015080) | |||||
| REF 88 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011425) | |||||
| REF 89 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020680) | |||||
| REF 90 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023897) | |||||
| REF 91 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000792) | |||||
| REF 92 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000885) | |||||
| REF 93 | Pharmacological characterization of a novel oestrogen antagonist, ZK 119010, in rats and mice. J Endocrinol. 1991 Sep;130(3):409-14. | |||||
| REF 94 | Benzothiophene selective estrogen receptor modulators with modulated oxidative activity and receptor affinity. J Med Chem. 2007 May 31;50(11):2682-92. | |||||
| REF 95 | Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause. 2009 Nov-Dec;16(6):1109-15. | |||||
| REF 96 | Estrogen replacement therapy and cardioprotection: mechanisms and controversies. Braz J Med Biol Res. 2002 Mar;35(3):271-6. | |||||
| REF 97 | Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med. 1997 Dec 8-22;157(22):2609-15. | |||||
| REF 98 | The future of the new selective estrogen receptor modulators. Menopause Int. 2007 Mar;13(1):27-34. | |||||
| REF 99 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 100 | Effect of MK-486 alone on Parkinsonism; studies on the clinical symptoms and catecholamine concentrations in the blood, urine and cerebrospinal fluid. No To Shinkei. 1977 Feb;29(2):205-13. | |||||
| REF 101 | Fluorine-substituted cyclofenil derivatives as estrogen receptor ligands: synthesis and structure-affinity relationship study of potential positron emission tomography agents for imaging estrogen receptors in breast cancer. J Med Chem. 2006 Apr 20;49(8):2496-511. | |||||
| REF 102 | Danazol suppression of luteinizing hormone in the rat: evidence for mediation by both androgen and estrogen receptors. Proc Soc Exp Biol Med. 1990 May;194(1):54-7. | |||||
| REF 103 | Potential use of an estrogen-glucocorticoid receptor chimera as a drug screen for tissue selective estrogenic activity. Bone. 2009 Jan;44(1):102-12. | |||||
| REF 104 | Effects of diethylstilbestrol on programmed oocyte death and induction of polyovular follicles in neonatal mouse ovaries. Biol Reprod. 2009 Nov;81(5):1002-9. | |||||
| REF 105 | RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs. 2015 Oct;26(9):948-56. | |||||
| REF 106 | Safety and efficacy of low-dose esterified estrogens and methyltestosterone, alone or combined, for the treatment of hot flashes in menopausal women: a randomized, double-blind, placebo-controlled study. Fertil Steril. 2011 Jan;95(1):366-8. | |||||
| REF 107 | Reprint of Are all estrogens the same Maturitas. 2008 Sep-Oct;61(1-2):195-201. | |||||
| REF 108 | Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med. 2002 Mar 28;346(13):967-74. | |||||
| REF 109 | US patent application no. 5,004,651, Stabilizing system for solid dosage forms. | |||||
| REF 110 | 17alpha-Ethinylestradiol hinders nucleotide excision repair in zebrafish liver cells. Aquat Toxicol. 2009 Dec 13;95(4):273-8. | |||||
| REF 111 | Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009 Jul;109(7):3012-43. | |||||
| REF 112 | Assay of the synthetic estrogen fosfestrol in pharmaceutical formulations using capillary electrophoresis. J Pharm Biomed Anal. 2005 Sep 15;39(3-4):559-63. | |||||
| REF 113 | Centchroman, a selective estrogen receptor modulator, as a contraceptive and for the management of hormone-related clinical disorders. Med Res Rev. 2001 Jul;21(4):302-47. | |||||
| REF 114 | Short-term effects of environmentally relevant concentrations of EDC mixtures on Mytilus galloprovincialis digestive gland. Aquat Toxicol. 2008 May 30;87(4):272-9. | |||||
| REF 115 | Mitotane for adrenocortical carcinoma treatment. Curr Opin Investig Drugs. 2005 Apr;6(4):386-94. | |||||
| REF 116 | Mitotane has an estrogenic effect on sex hormone-binding globulin and corticosteroid-binding globulin in humans. J Clin Endocrinol Metab. 2006 Jun;91(6):2165-70. | |||||
| REF 117 | Clinical pipeline report, company report or official report of Shionogi (2011). | |||||
| REF 118 | The Haunting of Medical Journals: How Ghostwriting Sold RT PLoS Med. 2010 September; 7(9): e1000335. | |||||
| REF 119 | Promestriene, a specific topic estrogen. Review of 40 years of vaginal atrophy treatment: is it safe even in cancer patients. Anticancer Drugs. 2013 Nov;24(10):989-98. | |||||
| REF 120 | The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone. 2007 Mar;40(3):674-84. | |||||
| REF 121 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 620). | |||||
| REF 122 | Modulators of vascular sex hormone receptors and their effects in estrogen-deficiency states associated with menopause. Recent Pat Cardiovasc Drug Discov. 2008 Nov;3(3):165-86. | |||||
| REF 123 | Chemoprevention for high-risk women: tamoxifen and beyond. Breast J. 2001 Sep-Oct;7(5):311-20. | |||||
| REF 124 | Effect of selective estrogen receptor modulators on cell proliferation and estrogen receptor activities in normal human prostate stromal and epithe... Prostate Cancer Prostatic Dis. 2009;12(4):375-81. | |||||
| REF 125 | Bioactivation of the selective estrogen receptor modulator acolbifene to quinone methides. Chem Res Toxicol. 2005 Feb;18(2):174-82. | |||||
| REF 126 | GDC-9545 (Giredestrant): A Potent and Orally Bioavailable Selective Estrogen Receptor Antagonist and Degrader with an Exceptional Preclinical Profile for ER+ Breast Cancer. J Med Chem. 2021 Aug 26;64(16):11841-11856. | |||||
| REF 127 | NM101 Phase III study of NE3107 in Alzheimer's disease: rationale, design?and therapeutic modulation of neuroinflammation and insulin resistance. Neurodegener Dis Manag. 2021 Aug;11(4):289-298. | |||||
| REF 128 | Relief of hot flushes with new plant-derived 10-component synthetic conjugated estrogens. Obstet Gynecol. 2004 Feb;103(2):245-53. | |||||
| REF 129 | Differential effects of estrogens and progestins on the anticoagulant tissue factor pathway inhibitor in the rat. J Steroid Biochem Mol Biol. 2005 Mar;94(4):361-8. | |||||
| REF 130 | Clinical pipeline report, company report or official report of Pharmabiz. | |||||
| REF 131 | Identification of GDC-0810 (ARN-810), an Orally Bioavailable Selective Estrogen Receptor Degrader (SERD) that Demonstrates Robust Activity in Tamoxifen-Resistant Breast Cancer Xenografts. J Med Chem.2015 Jun 25;58(12):4888-904. | |||||
| REF 132 | Discovery of AZD9833, a Potent and Orally Bioavailable Selective Estrogen Receptor Degrader and Antagonist. J Med Chem. 2020 Dec 10;63(23):14530-14559. | |||||
| REF 133 | Clinical pipeline report, company report or official report of Pantarhei Bioscience. | |||||
| REF 134 | Selective estrogen receptor alpha agonist GTx-758 decreases testosterone with reduced side effects of androgen deprivation therapy in men with advanced prostate cancer. Eur Urol. 2015 Feb;67(2):334-41. | |||||
| REF 135 | A novel prenylflavone restricts breast cancer cell growth through AhR-mediated destabilization of ER protein.Carcinogenesis.2012 May;33(5):1089-97. | |||||
| REF 136 | National Cancer Institute Drug Dictionary (drug id 599816). | |||||
| REF 137 | Clinical pipeline report, company report or official report of Olema Pharmaceuticals. | |||||
| REF 138 | Clinical pipeline report, company report or official report of Zentalis Pharmaceuticals. | |||||
| REF 139 | Clinical pipeline report, company report or official report of Accutar Biotechnology | |||||
| REF 140 | Agents with selective estrogen receptor (ER) modulator activity induce apoptosis in vitro and in vivo in ER-negative glioma cells. Cancer Res. 2004 Dec 15;64(24):9115-23. | |||||
| REF 141 | Pharmacological actions of a novel, potent, tissue-selective benzopyran estrogen. J Pharmacol Exp Ther. 2002 Oct;303(1):196-203. | |||||
| REF 142 | Clinical pipeline report, company report or official report of InventisBio. | |||||
| REF 143 | G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer. Breast Cancer Res Treat. 2020 Apr;180(3):635-646. | |||||
| REF 144 | Clinical pipeline report, company report or official report of Sun Pharma Advanced Research Company. | |||||
| REF 145 | Clinical pipeline report, company report or official report of TTC Oncology. | |||||
| REF 146 | In silico identification and biochemical evaluation of novel inhibitors of NRH:quinone oxidoreductase 2 (NQO2). Bioorg Med Chem Lett. 2010 Dec 15;20(24):7331-6. | |||||
| REF 147 | Inactivation of the uterine estrogen receptor binding of estradiol during P-450 catalyzed metabolism of chlorotrianisene (TACE). Speculation that TACE antiestrogenic activity involves covalent binding to the estrogen receptor. FEBS Lett. 1990 Feb 12;261(1):59-62. | |||||
| REF 148 | Bone targeted drugs 2. synthesis of estrogens with hydroxyapatite affinity, Bioorg. Med. Chem. Lett. 6(9):1047-1050 (1996). | |||||
| REF 149 | EM-800, a novel antiestrogen, acts as a pure antagonist of the transcriptional functions of estrogen receptors alpha and beta. Endocrinology. 1998 Jan;139(1):111-8. | |||||
| REF 150 | Idoxifene: a novel selective estrogen receptor modulator prevents bone loss and lowers cholesterol levels in ovariectomized rats and decreases uterine weight in intact rats. Endocrinology. 1998 Dec;139(12):5224-34. | |||||
| REF 151 | Estrogen agonistic/antagonistic effects of miproxifene phosphate (TAT-59)Shibata J1, Toko T, Saito H, Lykkesfeldt AE, Fujioka A, Sato K, Hashimoto A, Wierzba K, Yamada Y.Author information1Hanno Research Center, Taiho Pharmaceutical Co., Ltd., 1-27 Misugidai, Hanno-City, Saitama, 357-8527, Japan.Erratum inCancer Chemother Pharmacol 2000;46(2):172. AbstractPURPOSE: We evaluated miproxifene phosphate (TAT-59) to elucidate its efficacy in antiestrogen therapy for breast cancer patients and to assess its tissue-selective estrogenic/antiestrogenic activity.METHODS: Using DP-TAT-59, a major and active metabolite of TAT-59, an in vitro cell growth inhibition test was performed. Antitumor activity was determined using TAT-59 against human tumor xenografts of the MCF-7 and the Br-10 cell lines andMCF-7-derived tamoxifen-resistant cell lines, R-27 and FST-1. The antitumor activity of DP-TAT-59 and DM-DP-TAT-59, major metabolites of TAT-59 found in human blood following a TAT-59 dose, was also examined after intravenous administration to experimental animals. The residual estrogenic activity of TAT-59, evaluated in terms of bone and lipid metabolism in ovariectomized rats, was then comparedwith that of tamoxifen.RESULTS: DP-TAT-59 significantly inhibited the proliferation of estrogen receptor-positive MCF-7 and T-47D tumor cells in the presence of 1 nM estradiol. TAT-59, given to mice bearing MCF-7 or Br-10 xenografts, at the dose level of 5 mg/kg, exerted a significant growth inhibitory effect that was stronger than that of tamoxifen. Moreover, R-27 and FST-1 tumors, which show a resistance to tamoxifen, responded strongly to TAT-59, suggesting that TAT-59 might be effective against tumors resistant to tamoxifen. The metabolites of TAT-59, DP-TAT-59 and DM-DP-TAT-59, showed similar antitumor activity. Both TAT-59 and tamoxifen suppressed the decrease in bone density and reduced the blood cholesterol levels in ovariectomized rats, suggesting that the estrogenic activity of TAT-59 is comparable to that of tamoxifen.CONCLUSIONS: On the basis of the above results, one may expect TAT-59 to become an effective drug in patients with tumors less sensitive to tamoxifen, while its estrogenic activity as determined by bone and lipid metabolism is similar to that of tamoxifen.PMID: 10663628 [PubMed - indexed for MEDLINE] ShareMeSH Terms, SubstancesMeSH TermsAnimalsAntineoplastic Agents, Hormonal/pharmacologyBreast Neoplasms/pathology*Cell Division/drug effectsDrug Screening Assays, AntitumorEstradiol/pharmacologyEstrogen Antagonists/pharmacology*FemaleHumansLipid MetabolismMiceMice, Inbred BALB CRatsRats, Sprague-DawleyReceptors, Estrogen/physiologyTamoxifen/analogs & derivatives*Tamoxifen/pharmacologyTransplantation, HeterologousTumor Cells, Cultured/drug effectsSubstancesAntineoplastic Agents, HormonalEstrogen AntagonistsReceptors, EstrogenTamoxifenTAT 59EstradiolLinkOut - more resourcesOther Literature SourcesAccess more work from the authors - ResearchGateMedicalBreast Cancer - MedlinePlus Health InformationMiscellaneousESTRADIOL - Hazardous Substances Data BankTAMOXIFEN - Hazardous Substances Data BankPubMed Commons home Cancer Chemother Pharmacol. 2000;45(2):133-41. | |||||
| REF 152 | Droloxifene, a new antiestrogen: its role in metastatic breast cancer. Breast Cancer Res Treat. 1994;31(1):83-94. | |||||
| REF 153 | Combination therapy for treating breast cancer using antiestrogen, ERA-923, and the mammalian target of rapamycin inhibitor, temsirolimus. Endocr Relat Cancer. 2006 Sep;13(3):863-73. | |||||
| REF 154 | Design, synthesis, and preclinical characterization of novel, highly selective indole estrogens. J Med Chem. 2001 May 24;44(11):1654-7. | |||||
| REF 155 | Therapeutic targets in dry eye syndrome. Drug News Perspect. 2008 Apr;21(3):166-76. | |||||
| REF 156 | Species differences in metabolism of panomifene, an analogue of tamoxifen. Drug Metab Dispos. 1997 Dec;25(12):1370-8. | |||||
| REF 157 | CN patent application no. 102406650, Sex steroid precursors alone or in combination with selective estrogen receptor modulator for prevention and treatment of vaginal dryness and sexual dysfunction in postmenop. | |||||
| REF 158 | US patent application no. 2010,0278,784, Methods and compositions for treating skin conditions. | |||||
| REF 159 | Androstene-3,5-dienes as ER-beta selective SERMs. Bioorg Med Chem Lett. 2007 Nov 15;17(22):6295-8. | |||||
| REF 160 | US patent application no. 2010,0278,784, Methods and compositions for treating skin conditions. | |||||
| REF 161 | Review of the effects of 17alpha-estradiol in humans: a less feminizing estrogen with neuroprotective potential. Article first published online: 9 FEB 2009. | |||||
| REF 162 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023897) | |||||
| REF 163 | Clinical pipeline report, company report or official report of Bionovo. | |||||
| REF 164 | Synthesis and biological activity of new halo-steroidal antiestrogens. J Med Chem. 1991 May;34(5):1624-30. | |||||
| REF 165 | Structure-activity relationship of antiestrogens. Phenolic analogues of 2,3-diaryl-2H-1-benzopyrans. J Med Chem. 1990 Dec;33(12):3222-9. | |||||
| REF 166 | Genomic action of permanently charged tamoxifen derivatives via estrogen receptor-alpha. Bioorg Med Chem. 2010 Aug 1;18(15):5593-601. | |||||
| REF 167 | Phase I/II study of the anti-oestrogen zindoxifene (D16726) in the treatment of advanced breast cancer. A Cancer Research Campaign Phase I/II Clinical Trials Committee study.. Br J Cancer. 1990 March; 61(3): 451-453. | |||||
| REF 168 | 2-Phenylindole-linked [2-(aminoalkyl)pyridine]dichloroplatinum(II): complexes with a selective action on estrogen receptor positive mammary tumors. J Med Chem. 1991 Jul;34(7):2145-52. | |||||
| REF 169 | Differential response of estrogen receptor subtypes to 1,3-diarylindene and 2,3-diarylindene ligands. J Med Chem. 2005 Sep 22;48(19):5989-6003. | |||||
| REF 170 | ERbeta ligands. 3. Exploiting two binding orientations of the 2-phenylnaphthalene scaffold to achieve ERbeta selectivity. J Med Chem. 2005 Jun 16;48(12):3953-79. | |||||
| REF 171 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 172 | Estrogen receptor beta selective ligands: discovery and SAR of novel heterocyclic ligands. Bioorg Med Chem Lett. 2005 Dec 15;15(24):5562-6. | |||||
| REF 173 | Blocking estrogen signaling after the hormone: pyrimidine-core inhibitors of estrogen receptor-coactivator binding. J Med Chem. 2008 Oct 23;51(20):6512-30. | |||||
| REF 174 | Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-beta ligands. J Med Chem. 2004 Oct 7;47(21):5021-40. | |||||
| REF 175 | 2-Phenylspiroindenes: a novel class of selective estrogen receptor modulators (SERMs). Bioorg Med Chem Lett. 2003 Feb 10;13(3):479-83. | |||||
| REF 176 | ERbeta ligands. Part 4: Synthesis and structure-activity relationships of a series of 2-phenylquinoline derivatives. Bioorg Med Chem Lett. 2005 Oct 15;15(20):4520-5. | |||||
| REF 177 | 7-Substituted 2-phenyl-benzofurans as ER beta selective ligands. Bioorg Med Chem Lett. 2004 Oct 4;14(19):4925-9. | |||||
| REF 178 | Phenolic metabolites of clomiphene: [(E,Z)-2-[4-(1,2-diphenyl-2-chlorovinyl)phenoxy]ethyl]diethylamine. Preparation, electrophilicity, and effects ... J Med Chem. 1989 Jan;32(1):192-7. | |||||
| REF 179 | 6H-Benzo[c]chromen-6-one derivatives as selective ERbeta agonists. Bioorg Med Chem Lett. 2006 Mar 15;16(6):1468-72. | |||||
| REF 180 | Effects of 7-O substitutions on estrogenic and anti-estrogenic activities of daidzein analogues in MCF-7 breast cancer cells. J Med Chem. 2010 Aug 26;53(16):6153-63. | |||||
| REF 181 | Monoaryl-substituted salicylaldoximes as ligands for estrogen receptor beta. J Med Chem. 2008 Mar 13;51(5):1344-51. | |||||
| REF 182 | Influence of alkyl chain ramification on estradiol receptor binding affinity and intrinsic activity of 1,2-dialkylated 1,2-bis(4- or 3-hydroxypheny... J Med Chem. 1986 Sep;29(9):1668-74. | |||||
| REF 183 | Isolation and structure elucidation of an isoflavone and a sesterterpenoic acid from Henriettella fascicularis. J Nat Prod. 2002 Dec;65(12):1749-53. | |||||
| REF 184 | Investigations on estrogen receptor binding. The estrogenic, antiestrogenic, and cytotoxic properties of C2-alkyl-substituted 1,1-bis(4-hydroxyphen... J Med Chem. 2002 Nov 21;45(24):5358-64. | |||||
| REF 185 | Synthesis and activity of substituted 4-(indazol-3-yl)phenols as pathway-selective estrogen receptor ligands useful in the treatment of rheumatoid ... J Med Chem. 2004 Dec 16;47(26):6435-8. | |||||
| REF 186 | Substituted 4-hydroxyphenyl sulfonamides as pathway-selective estrogen receptor ligands. Bioorg Med Chem Lett. 2006 Feb 15;16(4):854-8. | |||||
| REF 187 | Antiestrogenically active 1,1,2-tris(4-hydroxyphenyl)alkenes without basic side chain: synthesis and biological activity. J Med Chem. 2003 Apr 10;46(8):1484-91. | |||||
| REF 188 | Estrogen receptor beta ligands: design and synthesis of new 2-phenyl-isoindole-1,3-diones. Bioorg Med Chem Lett. 2007 Jan 1;17(1):118-22. | |||||
| REF 189 | Subtle side-chain modifications of the hop phytoestrogen 8-prenylnaringenin result in distinct agonist/antagonist activity profiles for estrogen re... J Med Chem. 2006 Dec 14;49(25):7357-65. | |||||
| REF 190 | New estrogenic compounds isolated from Broussonetia kazinoki. Bioorg Med Chem Lett. 2010 Jun 15;20(12):3764-7. | |||||
| REF 191 | Utility of boron clusters for drug design. Relation between estrogen receptor binding affinity and hydrophobicity of phenols bearing various types ... Bioorg Med Chem Lett. 2003 Nov 17;13(22):4089-92. | |||||
| REF 192 | Structure-based virtual screening for plant-based ERbeta-selective ligands as potential preventative therapy against age-related neurodegenerative ... J Med Chem. 2005 May 19;48(10):3463-6. | |||||
| REF 193 | Discovery of potent, nonsteroidal, and highly selective glucocorticoid receptor antagonists. J Med Chem. 2002 Jun 6;45(12):2417-24. | |||||
| REF 194 | Synthesis and biological activity of trans-2,3-dihydroraloxifene. Bioorg Med Chem Lett. 1999 Apr 19;9(8):1137-40. | |||||
| REF 195 | Design, synthesis, and biological evaluation of doxorubicin-formaldehyde conjugates targeted to breast cancer cells. J Med Chem. 2004 Feb 26;47(5):1193-206. | |||||
| REF 196 | Estrogen: estrone (E1), estradiol (E2), estriol (E3) and estetrol (E4). Nihon Rinsho. 2010 Jul;68 Suppl 7:448-61. | |||||
| REF 197 | Synthesis and evaluation of geldanamycin-estradiol hybrids. Bioorg Med Chem Lett. 1999 May 3;9(9):1233-8. | |||||
| REF 198 | Structure-guided synthesis of tamoxifen analogs with improved selectivity for the orphan ERRgamma. Bioorg Med Chem Lett. 2006 Feb 15;16(4):821-4. | |||||
| REF 199 | Molecular mechanism of action at estrogen receptor alpha of a new clinically relevant antiestrogen (GW7604) related to tamoxifen. Endocrinology. 2001 Feb;142(2):838-46. | |||||
| REF 200 | Identification and structure-activity relationships of chromene-derived selective estrogen receptor modulators for treatment of postmenopausal symp... J Med Chem. 2009 Dec 10;52(23):7544-69. | |||||
| REF 201 | Bicyclo[2.2.2]octanes: close structural mimics of the nuclear receptor-binding motif of steroid receptor coactivators. Bioorg Med Chem Lett. 2007 Aug 1;17(15):4118-22. | |||||
| REF 202 | Antagonists selective for estrogen receptor alpha. Endocrinology. 2002 Mar;143(3):941-7. | |||||
| REF 203 | Analogs of methyl-piperidinopyrazole (MPP): antiestrogens with estrogen receptor alpha selective activity. Bioorg Med Chem Lett. 2009 Jan 1;19(1):108-10. | |||||
| REF 204 | Discovery and preclinical pharmacology of a novel, potent, nonsteroidal estrogen receptor agonist/antagonist, CP-336156, a diaryltetrahydronaphthal... J Med Chem. 1998 Jul 30;41(16):2928-31. | |||||
| REF 205 | The selective estrogen receptor alpha agonist Org 37663 induces estrogenic effects but lacks antirheumatic activity: a phase IIa trial investigating efficacy and safety of Org 37663 in postmenopausal female rheumatoid arthritis patients receiving stable background methotrexate or sulfasalazine.Arthritis Rheum.2010 Feb;62(2):351-8. | |||||
| REF 206 | Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-alpha and estrogen receptor-beta: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology. 2000 Oct;141(10):3534-45. | |||||
| REF 207 | Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology. 1999 Feb;140(2):800-4. | |||||
| REF 208 | Synthesis and biological evaluation of stilbene-based pure estrogen antagonists. Bioorg Med Chem Lett. 2004 Sep 20;14(18):4659-63. | |||||
| REF 209 | Novel estrogen receptor ligands based on an anthranylaldoxime structure: role of the phenol-type pseudocycle in the binding process. J Med Chem. 2003 Sep 11;46(19):4032-42. | |||||
| REF 210 | Stereospecific lasofoxifene derivatives reveal the interplay between estrogen receptor alpha stability and antagonistic activity in ESR1 mutant breast cancer cells. Elife. 2022 May 16;11:e72512. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

