Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DATO92
|
|||
| Drug Name |
AZD9833
|
|||
| Synonyms |
AZD-9833; UNII-JUP57A8EPZ; JUP57A8EPZ; 2222844-89-3; Camizestrant; Camizestrant [USAN]; SCHEMBL20089710; NSC828717; WHO 11592; NSC-828717; HY-136255; AZ14066724; CS-0121043; 3-Pyridinamine, N-(1-(3-fluoropropyl)-3-azetidinyl)-6-((6S,8R)-6,7,8,9-tetrahydro-8-methyl-7-(2,2,2-trifluoroethyl)-3H-pyrazolo(4,3-F)isoquinolin-6-yl)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | ER-positive breast cancer [ICD-11: 2C60-2C65] | Phase 2 | [1] | |
| Company |
AstraZeneca
|
|||
| Structure |
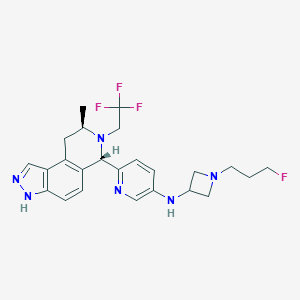 |
Download2D MOL |
||
| Formula |
C24H28F4N6
|
|||
| Canonical SMILES |
CC1CC2=C(C=CC3=C2C=NN3)C(N1CC(F)(F)F)C4=NC=C(C=C4)NC5CN(C5)CCCF
|
|||
| InChI |
1S/C24H28F4N6/c1-15-9-19-18(4-6-21-20(19)11-30-32-21)23(34(15)14-24(26,27)28)22-5-3-16(10-29-22)31-17-12-33(13-17)8-2-7-25/h3-6,10-11,15,17,23,31H,2,7-9,12-14H2,1H3,(H,30,32)/t15-,23+/m1/s1
|
|||
| InChIKey |
WDHOIABIERMLGY-CMJOXMDJSA-N
|
|||
| CAS Number |
CAS 2222844-89-3
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04214288) A Comparative Study of AZD9833 Versus Fulvestrant in Women With Advanced ER-Positive HER2-Negative Breast Cancer (SERENA-2). U.S. National Institutes of Health. | |||
| REF 2 | Discovery of AZD9833, a Potent and Orally Bioavailable Selective Estrogen Receptor Degrader and Antagonist. J Med Chem. 2020 Dec 10;63(23):14530-14559. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

