Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0SY2M
|
|||
| Former ID |
DIB019692
|
|||
| Drug Name |
daidzein
|
|||
| Synonyms |
Daidzein; 486-66-8; 4',7-Dihydroxyisoflavone; Daidzeol; 7,4'-Dihydroxyisoflavone; 7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one; 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-; 7-Hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 7-hydroxy-3-(4-hydroxyphenyl)chromen-4-one; 7-Hydroxy-3-(4-hydroxyphenyl)-4-benzopyrone; UNII-6287WC5J2L; CCRIS 7600; K 251b; 4,7-Dihydroxyisoflavone; EINECS 207-635-4; BRN 0231523; CHEMBL8145; 4',7-Dihydroxy-iso-flavone; d-(+)-alpha-methylbenzylamine; ,7-Dihydroxyisoflavone; 7,4'-dihydroxyisoflavone; DIADZEIN
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2] | |
| Structure |
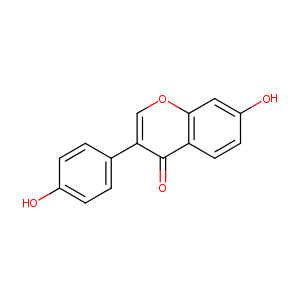 |
Download2D MOL |
||
| Formula |
C15H10O4
|
|||
| Canonical SMILES |
C1=CC(=CC=C1C2=COC3=C(C2=O)C=CC(=C3)O)O
|
|||
| InChI |
1S/C15H10O4/c16-10-3-1-9(2-4-10)13-8-19-14-7-11(17)5-6-12(14)15(13)18/h1-8,16-17H
|
|||
| InChIKey |
ZQSIJRDFPHDXIC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 486-66-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
12394, 623493, 5142892, 8144381, 8149155, 8616734, 11111095, 11114077, 11336198, 11361437, 11363149, 11365711, 11368273, 11371341, 11375568, 11376435, 11462409, 11483941, 11487869, 11490007, 11493778, 11494069, 11537432, 14720388, 14847806, 17389519, 17404975, 22391461, 24278030, 25622055, 26527426, 26613045, 26679806, 26747166, 26747167, 26752188, 26752189, 26758540, 29204010, 39315324, 46487931, 46500432, 47213225, 47216588, 47290947, 47440044, 47440045, 47440046, 47515129, 47959544
|
|||
| ChEBI ID |
CHEBI:28197
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Coriobacteriales | ||||
|
Studied Microbe: Anaerobic bacterium Mt1B8
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Resulting Metabolite | Dihydrodaidzein; equol | |||
| Metabolic Effect | Increase activity | |||
| Description | Daidzein can be metabolized to Dihydrodaidzein and equol by Anaerobic bacterium Mt1B8, which results in the increase of the drug's activity. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eggerthellales | ||||
|
Studied Microbe: Adlercreutzia equolifaciens
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Resulting Metabolite | 5-hydroxy-equol | |||
| Description | Daidzein can be metabolized to 5-hydroxy-equol by Adlercreutzia equolifaciens. | |||
|
Studied Microbe: Slackia equolifaciens
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Resulting Metabolite | 5-hydroxy-equol | |||
| Description | Daidzein can be metabolized to 5-hydroxy-equol by Slackia equolifaciens. | |||
|
Studied Microbe: Slackia isoflavoniconvertens
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Resulting Metabolite | 5-hydroxy-equol | |||
| Description | Daidzein can be metabolized to 5-hydroxy-equol by Slackia isoflavoniconvertens. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Clostridium sp.
Show/Hide Hierarchy
|
[5], [6], [7] | |||
| Hierarchy | ||||
| Metabolic Reaction | Reduction and phenolic ring opening | |||
| Resulting Metabolite | Dihydrodaidzein, equol; O-desmethylanolensin | |||
| Metabolic Effect | Increase activity | |||
| Description | Daidzein can be metabolized to Dihydrodaidzein, equol and O-desmethylanolensin by Clostridium sp. through reduction and phenolic ring opening, which results in the increase of the drug's activity. | |||
|
Studied Microbe: Eubacterium ramulus
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Description | Daidzein can be metabolized by Eubacterium ramulus. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Lactobacillales | ||||
|
Studied Microbe: Lactococcus garvieae
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Resulting Metabolite | 5-hydroxy-equol | |||
| Description | Daidzein can be metabolized to 5-hydroxy-equol by Lactococcus garvieae. | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
|
Studied Microbe: Anaerobic bacterium 'strain 7'
Show/Hide Hierarchy
|
[5] | |||
| Hierarchy | ||||
| Metabolic Reaction | Reduction and phenolic ring opening | |||
| Resulting Metabolite | Dihydrodaidzein, equol; O-desmethylanolensin | |||
| Metabolic Effect | Increase activity | |||
| Description | Daidzein can be metabolized to Dihydrodaidzein, equol and O-desmethylanolensin by Anaerobic bacterium 'strain 7' through reduction and phenolic ring opening, which results in the increase of the drug's activity. | |||
| Studied Microbe: Anaerobic bacterium unspecific | [5] | |||
| Metabolic Reaction | Reduction and phenolic ring opening | |||
| Resulting Metabolite | Dihydrodaidzein, equol; O-desmethylanolensin | |||
| Metabolic Effect | Increase activity | |||
| Description | Daidzein can be metabolized to Dihydrodaidzein, equol and O-desmethylanolensin by unspecific Anaerobic bacterium through reduction and phenolic ring opening, which results in the increase of the drug's activity. | |||
| Studied Microbe: Gut microbiota unspecific | [8] | |||
| Resulting Metabolite | Dihydrodaidzein; equol | |||
| Metabolic Effect | Increase activity | |||
| Description | Daidzein can be metabolized to Dihydrodaidzein and equol by gut microbiota, which results in the increase of the drug's activity. | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | The balance between concurrent activation of ERs and PPARs determines daidzein-induced osteogenesis and adipogenesis. J Bone Miner Res. 2004 May;19(5):853-61. | |||
| REF 2 | Isolation and structure elucidation of an isoflavone and a sesterterpenoic acid from Henriettella fascicularis. J Nat Prod. 2002 Dec;65(12):1749-53. | |||
| REF 3 | Pharmacomicrobiomics: The Holy Grail to Variability in Drug Response?. Clin Pharmacol Ther. 2019 Aug;106(2):317-328. | |||
| REF 4 | Gut Microbiota-Mediated Drug-Antibiotic Interactions. Drug Metab Dispos. 2015 Oct;43(10):1581-9. | |||
| REF 5 | The influence of gut microbiota on drug metabolism and toxicity. Expert Opin Drug Metab Toxicol. 2016;12(1):31-40. | |||
| REF 6 | Gut microbiota: what is its place in pharmacology?. Expert Rev Clin Pharmacol. 2019 Oct;12(10):921-930. | |||
| REF 7 | Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch Microbiol. 2002 Jul;178(1):8-12. | |||
| REF 8 | The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008 Nov 3;363(1-2):1-25. | |||
| REF 9 | 1-Benzopyran-4-one antioxidants as aldose reductase inhibitors. J Med Chem. 1999 Jun 3;42(11):1881-93. | |||
| REF 10 | Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003 Nov;1(13):981-91. | |||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 490). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

