Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T26623
(Former ID: TTDS00115)
|
|||||
| Target Name |
Aldose reductase (AKR1B1)
|
|||||
| Synonyms |
Aldehyde reductase; AKR1B1
Click to Show/Hide
|
|||||
| Gene Name |
AKR1B1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Neuropathy [ICD-11: 8C0Z] | |||||
| 2 | Rheumatoid arthritis [ICD-11: FA20] | |||||
| Function |
Catalyzes the NADPH-dependent reduction of a wide variety of carbonyl-containing compounds to their corresponding alcohols with a broad range of catalytic efficiencies.
Click to Show/Hide
|
|||||
| BioChemical Class |
Short-chain dehydrogenases reductase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.1.1.300
|
|||||
| Sequence |
MASRLLLNNGAKMPILGLGTWKSPPGQVTEAVKVAIDVGYRHIDCAHVYQNENEVGVAIQ
EKLREQVVKREELFIVSKLWCTYHEKGLVKGACQKTLSDLKLDYLDLYLIHWPTGFKPGK EFFPLDESGNVVPSDTNILDTWAAMEELVDEGLVKAIGISNFNHLQVEMILNKPGLKYKP AVNQIECHPYLTQEKLIQYCQSKGIVVTAYSPLGSPDRPWAKPEDPSLLEDPRIKAIAAK HNKTTAQVLIRFPMQRNLVVIPKSVTPERIAENFKVFDFELSSQDMTTLLSYNRNWRVCA LLSCTSHKDYPFHEEF Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T68NCI | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 2 Approved Drugs | + | ||||
| 1 | Epalrestat | Drug Info | Approved | Diabetic neuropathy | [2] | |
| 2 | Sulindac | Drug Info | Approved | Rheumatoid arthritis | [3], [4] | |
| Clinical Trial Drug(s) | [+] 11 Clinical Trial Drugs | + | ||||
| 1 | Fidarestat | Drug Info | Phase 3 | Diabetic complication | [5] | |
| 2 | Ranirestat | Drug Info | Phase 3 | Diabetic neuropathy | [6] | |
| 3 | BNV-222 | Drug Info | Phase 2/3 | Diabetic neuropathy | [7] | |
| 4 | CONTIGOSIDE B | Drug Info | Phase 2/3 | Thrombosis | [8] | |
| 5 | ADMVA | Drug Info | Phase 2 | Diabetic complication | [9], [10] | |
| 6 | LIDORESTAT | Drug Info | Phase 2 | Diabetic complication | [11], [12] | |
| 7 | M-16209 | Drug Info | Phase 2 | Diabetic complication | [13] | |
| 8 | QR-333 | Drug Info | Phase 2 | Diabetic neuropathy | [14] | |
| 9 | T2c-003 | Drug Info | Phase 1/2 | Diabetic neuropathy | [15] | |
| 10 | ALO-1567 | Drug Info | Phase 1 | Glaucoma/ocular hypertension | [16] | |
| 11 | SSR-125047 | Drug Info | Phase 1 | Schizophrenia | [17] | |
| Discontinued Drug(s) | [+] 13 Discontinued Drugs | + | ||||
| 1 | IMIRESTAT | Drug Info | Discontinued in Phase 3 | Diabetic complication | [18] | |
| 2 | MINALRESTAT | Drug Info | Discontinued in Phase 3 | Diabetic complication | [19] | |
| 3 | Ponalrestat | Drug Info | Discontinued in Phase 3 | Gout | [20] | |
| 4 | AD-5467 | Drug Info | Discontinued in Phase 2 | Diabetic complication | [21] | |
| 5 | CTL-102-GDEPT | Drug Info | Discontinued in Phase 2 | Head and neck cancer | [22] | |
| 6 | JTT-811 | Drug Info | Discontinued in Phase 2 | Diabetic complication | [23] | |
| 7 | ZOPOLRESTAT | Drug Info | Discontinued in Phase 2 | Diabetic complication | [24], [25] | |
| 8 | E-0722 | Drug Info | Terminated | Diabetic cataract | [27] | |
| 9 | FR-62765 | Drug Info | Terminated | Diabetic complication | [28] | |
| 10 | Sorbinil | Drug Info | Terminated | Diabetic cataract | [29], [30] | |
| 11 | SPR-210 | Drug Info | Terminated | Diabetic complication | [31] | |
| 12 | WF-2421 | Drug Info | Terminated | Diabetic complication | [32] | |
| 13 | Zenarestat | Drug Info | Terminated | Diabetic neuropathy | [33], [34] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | ARI-809 | Drug Info | Preclinical | Diabetic complication | [26] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 93 Inhibitor drugs | + | ||||
| 1 | Epalrestat | Drug Info | [35], [36] | |||
| 2 | Sulindac | Drug Info | [1], [37] | |||
| 3 | Fidarestat | Drug Info | [38], [39] | |||
| 4 | Ranirestat | Drug Info | [40] | |||
| 5 | BNV-222 | Drug Info | [7] | |||
| 6 | CONTIGOSIDE B | Drug Info | [41] | |||
| 7 | LIDORESTAT | Drug Info | [42] | |||
| 8 | M-16209 | Drug Info | [43], [44] | |||
| 9 | QR-333 | Drug Info | [45] | |||
| 10 | ALO-1567 | Drug Info | [16], [44] | |||
| 11 | SSR-125047 | Drug Info | [46] | |||
| 12 | IMIRESTAT | Drug Info | [47] | |||
| 13 | Ponalrestat | Drug Info | [49] | |||
| 14 | AD-5467 | Drug Info | [44], [50] | |||
| 15 | Alrestatin | Drug Info | [51] | |||
| 16 | JTT-811 | Drug Info | [40] | |||
| 17 | ARI-809 | Drug Info | [54] | |||
| 18 | Sorbinil | Drug Info | [56], [57] | |||
| 19 | SPR-210 | Drug Info | [58] | |||
| 20 | Zenarestat | Drug Info | [59] | |||
| 21 | (4-Methyl-2-oxo-2H-quinolin-1-yl)-acetic acid | Drug Info | [60] | |||
| 22 | (6-Hydroxy-2-oxo-2H-quinolin-1-yl)-acetic acid | Drug Info | [60] | |||
| 23 | (6-Methoxy-2-oxo-2H-quinolin-1-yl)-acetic acid | Drug Info | [60] | |||
| 24 | (8-Hydroxy-2-oxo-2H-quinolin-1-yl)-acetic acid | Drug Info | [60] | |||
| 25 | 2'-Monophosphoadenosine 5'-Diphosphoribose | Drug Info | [51] | |||
| 26 | 2,3-dihydroxypropanal | Drug Info | [61] | |||
| 27 | 2-(3,4-Dihydroxy-benzyl)-7-hydroxy-chromen-4-one | Drug Info | [62] | |||
| 28 | 2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one | Drug Info | [62] | |||
| 29 | 2-(3-benzoyl-1H-pyrrol-1-yl)acetic acid | Drug Info | [63] | |||
| 30 | 2-(4-aminophenylsulfonamido)acetic acid | Drug Info | [64] | |||
| 31 | 2-(Phenylsulfonamido)acetic Acid | Drug Info | [65] | |||
| 32 | 2-Benzhydryl-7-hydroxy-chromen-4-one | Drug Info | [62] | |||
| 33 | 2-Benzyl-7-hydroxy-chromen-4-one | Drug Info | [62] | |||
| 34 | 3,5-dichlorosalicylic acid | Drug Info | [66] | |||
| 35 | 3-(3-Benzoyl-1H-pyrrol-1-yl)propanoic acid | Drug Info | [63] | |||
| 36 | 3-[5-(3-nitrophenyl)thiophen-2-yl]propanoic acid | Drug Info | [67] | |||
| 37 | 4-(3-Benzoyl-1H-pyrrol-1-yl)butanoic acid | Drug Info | [63] | |||
| 38 | 4-(3-Methoxy-phenyl)-isoxazolidine-3,5-dione | Drug Info | [68] | |||
| 39 | 6,7-Dihydroxy-2-phenyl-chromen-4-one | Drug Info | [62] | |||
| 40 | 6-(1H-Indole-2-sulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 41 | 6-(2-Bromo-benzenesulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 42 | 6-(2-Chloro-benzenesulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 43 | 6-(2-Fluoro-benzenesulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 44 | 6-(3-Chloro-benzenesulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 45 | 6-(4-Bromo-benzenesulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 46 | 6-(4-Chloro-benzenesulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 47 | 6-(4-Fluoro-benzenesulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 48 | 6-(4-Methoxy-benzenesulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 49 | 6-(Benzofuran-2-sulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 50 | 6-(Benzothiazole-2-sulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 51 | 6-(Biphenyl-2-sulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 52 | 6-(Naphthalene-1-sulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 53 | 6-(Naphthalene-2-sulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 54 | 6-(Toluene-4-sulfonyl)-2H-pyridazin-3-one | Drug Info | [69] | |||
| 55 | 6-Benzenesulfonyl-2H-pyridazin-3-one | Drug Info | [69] | |||
| 56 | 6-Hydroxy-2-(4-hydroxy-benzyl)-chromen-4-one | Drug Info | [62] | |||
| 57 | 6-methoxykaempferol 3-O-beta-D-robinobioside | Drug Info | [70] | |||
| 58 | 6-Phenylmethanesulfonyl-2H-pyridazin-3-one | Drug Info | [69] | |||
| 59 | 7-Hydroxy-2-(4-hydroxy-benzyl)-chromen-4-one | Drug Info | [62] | |||
| 60 | 7-Hydroxy-2-(4-methoxy-benzyl)-chromen-4-one | Drug Info | [62] | |||
| 61 | 7-Hydroxy-4-phenylcoumarin | Drug Info | [71] | |||
| 62 | 7-Hydroxy-6-nitro-2-phenyl-chromen-4-one | Drug Info | [62] | |||
| 63 | AK198 | Drug Info | [72] | |||
| 64 | Alpha-D-Glucose-6-Phosphate | Drug Info | [51] | |||
| 65 | APIGENIN | Drug Info | [73] | |||
| 66 | Apigenin-7-O-beta-D-glucuronide | Drug Info | [73] | |||
| 67 | Apigenin-7-O-beta-D-glucuronide methyl ester | Drug Info | [73] | |||
| 68 | ASTRAGALIN | Drug Info | [73] | |||
| 69 | Chrysin | Drug Info | [74] | |||
| 70 | daidzein | Drug Info | [62] | |||
| 71 | EPALRESTATE | Drug Info | [73] | |||
| 72 | Fidarestat(Stereoisomer) | Drug Info | [51] | |||
| 73 | Hydroxydimethylarsine Oxide | Drug Info | [51] | |||
| 74 | IDD552 | Drug Info | [51] | |||
| 75 | IDD594 | Drug Info | [67] | |||
| 76 | Inhibitor Idd 384 | Drug Info | [51] | |||
| 77 | Isorhamnetin 3,7-disulfate | Drug Info | [41] | |||
| 78 | Isorhamnetin 3-O-rhamnoside | Drug Info | [75] | |||
| 79 | KAEMPFEROL | Drug Info | [73] | |||
| 80 | MANGIFERIN | Drug Info | [76] | |||
| 81 | N-Acetylalanine | Drug Info | [51] | |||
| 82 | NSC-94258 | Drug Info | [62] | |||
| 83 | O5-Acetyl-O7-nitrooxyethyl chrysin | Drug Info | [74] | |||
| 84 | O7-Nitrooxyethyl chrysin | Drug Info | [74] | |||
| 85 | PALBINONE | Drug Info | [77] | |||
| 86 | Patuletin 3-O-beta-D-galactoside | Drug Info | [70] | |||
| 87 | Patuletin 3-O-beta-D-robinobioside | Drug Info | [70] | |||
| 88 | Quercetin 3-O-neohesperidoside | Drug Info | [75] | |||
| 89 | QUERCITRIN | Drug Info | [73] | |||
| 90 | Tamarixetin 3-glucoside-7-sulfate | Drug Info | [41] | |||
| 91 | TINGENIN B | Drug Info | [76] | |||
| 92 | TINGENONE | Drug Info | [76] | |||
| 93 | TRIPTOCALLINE A | Drug Info | [76] | |||
| Modulator | [+] 8 Modulator drugs | + | ||||
| 1 | T2c-003 | Drug Info | [40] | |||
| 2 | MINALRESTAT | Drug Info | [48] | |||
| 3 | CTL-102-GDEPT | Drug Info | [52] | |||
| 4 | ZOPOLRESTAT | Drug Info | [53] | |||
| 5 | E-0722 | Drug Info | [55] | |||
| 6 | FR-62765 | Drug Info | [28] | |||
| 7 | WF-2421 | Drug Info | [32] | |||
| 8 | BNV-222 | Drug Info | [40] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Tolmetin | Ligand Info | |||||
| Structure Description | Crystal Structure of Human Aldose Reductase Complexed with Tolmetin | PDB:3S3G | ||||
| Method | X-ray diffraction | Resolution | 1.80 Å | Mutation | No | [78] |
| PDB Sequence |
MASRLLLNNG
9 AKMPILGLGT19 WKSPPGQVTE29 AVKVAIDVGY39 RHIDCAHVYQ49 NENEVGVAIQ 59 EKLREQVVKR69 EELFIVSKLW79 CTYHEKGLVK89 GACQKTLSDL99 KLDYLDLYLI 109 HWPTGFKPGK119 EFFPLDESGN129 VVPSDTNILD139 TWAAMEELVD149 EGLVKAIGIS 159 NFNHLQVEMI169 LNKPGLKYKP179 AVNQIECHPY189 LTQEKLIQYC199 QSKGIVVTAY 209 SPLGSPDRPW219 AKPEDPSLLE229 DPRIKAIAAK239 HNKTTAQVLI249 RFPMQRNLVV 259 IPKSVTPERI269 AENFKVFDFE279 LSSQDMTTLL289 SYNRNWRVCA299 LLSCTSHKDY 309 PFHEEF
|
|||||
|
|
||||||
| Ligand Name: Nitazoxanide | Ligand Info | |||||
| Structure Description | Aldose reductase complexed with a nitro compound | PDB:3V35 | ||||
| Method | X-ray diffraction | Resolution | 1.90 Å | Mutation | No | [79] |
| PDB Sequence |
MASRLLLNNG
9 AKMPILGLGT19 WKSPPGQVTE29 AVKVAIDVGY39 RHIDCAHVYQ49 NENEVGVAIQ 59 EKLREQVVKR69 EELFIVSKLW79 CTYHEKGLVK89 GACQKTLSDL99 KLDYLDLYLI 109 HWPTGFKPGK119 EFFPLDESGN129 VVPSDTNILD139 TWAAMEELVD149 EGLVKAIGIS 159 NFNHLQVEMI169 LNKPGLKYKP179 AVNQIECHPY189 LTQEKLIQYC199 QSKGIVVTAY 209 SPLGSPDRPW219 AKPEDPSLLE229 DPRIKAIAAK239 HNKTTAQVLI249 RFPMQRNLVV 259 IPKSVTPERI269 AENFKVFDFE279 LSSQDMTTLL289 SYNRNWRVCA299 LLSCTSHKDY 309 PFHEEF
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
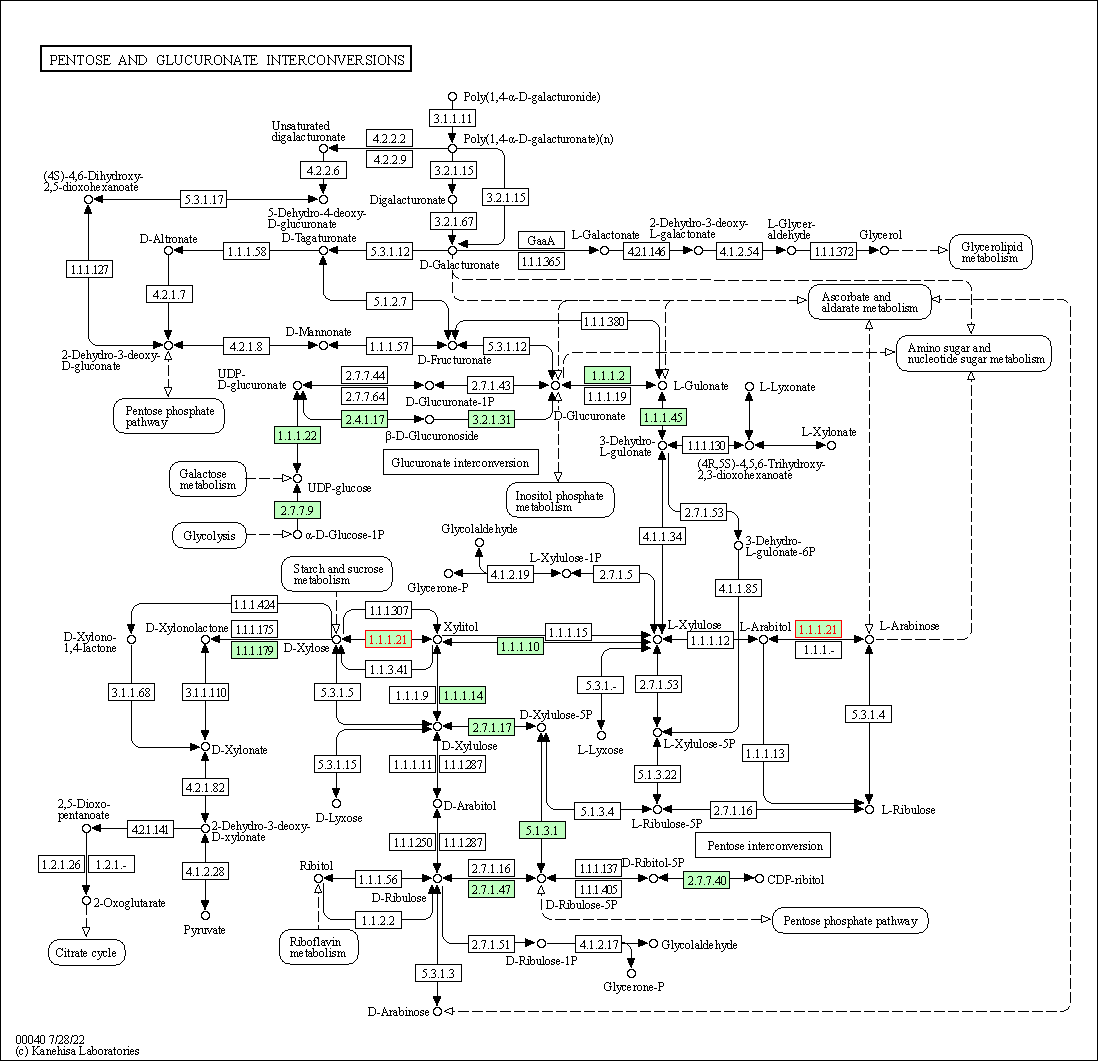
| Pentose and glucuronate interconversions | hsa00040 | Affiliated Target |

|
| Class: Metabolism => Carbohydrate metabolism | Pathway Hierarchy | ||
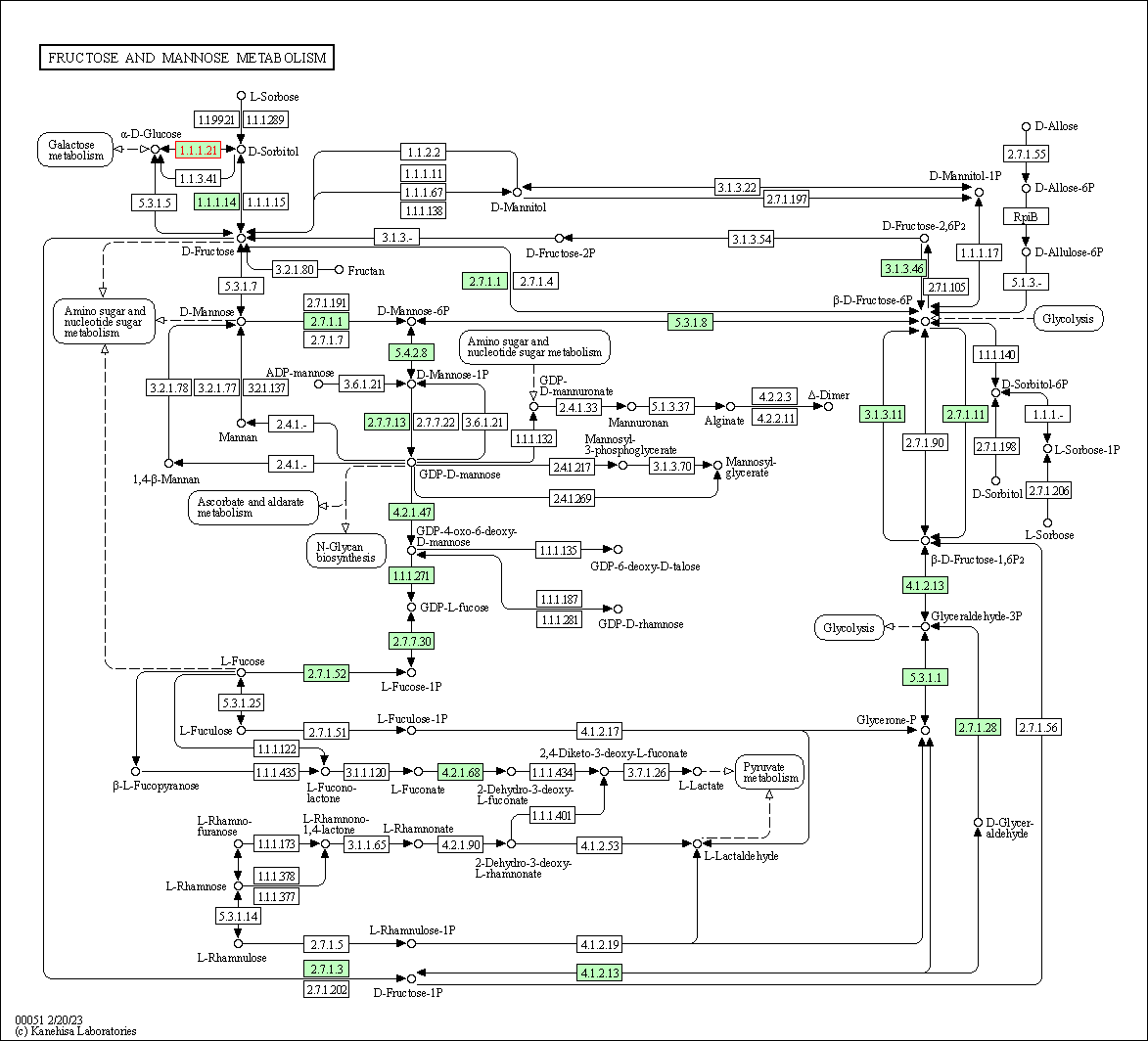
| Fructose and mannose metabolism | hsa00051 | Affiliated Target |

|
| Class: Metabolism => Carbohydrate metabolism | Pathway Hierarchy | ||
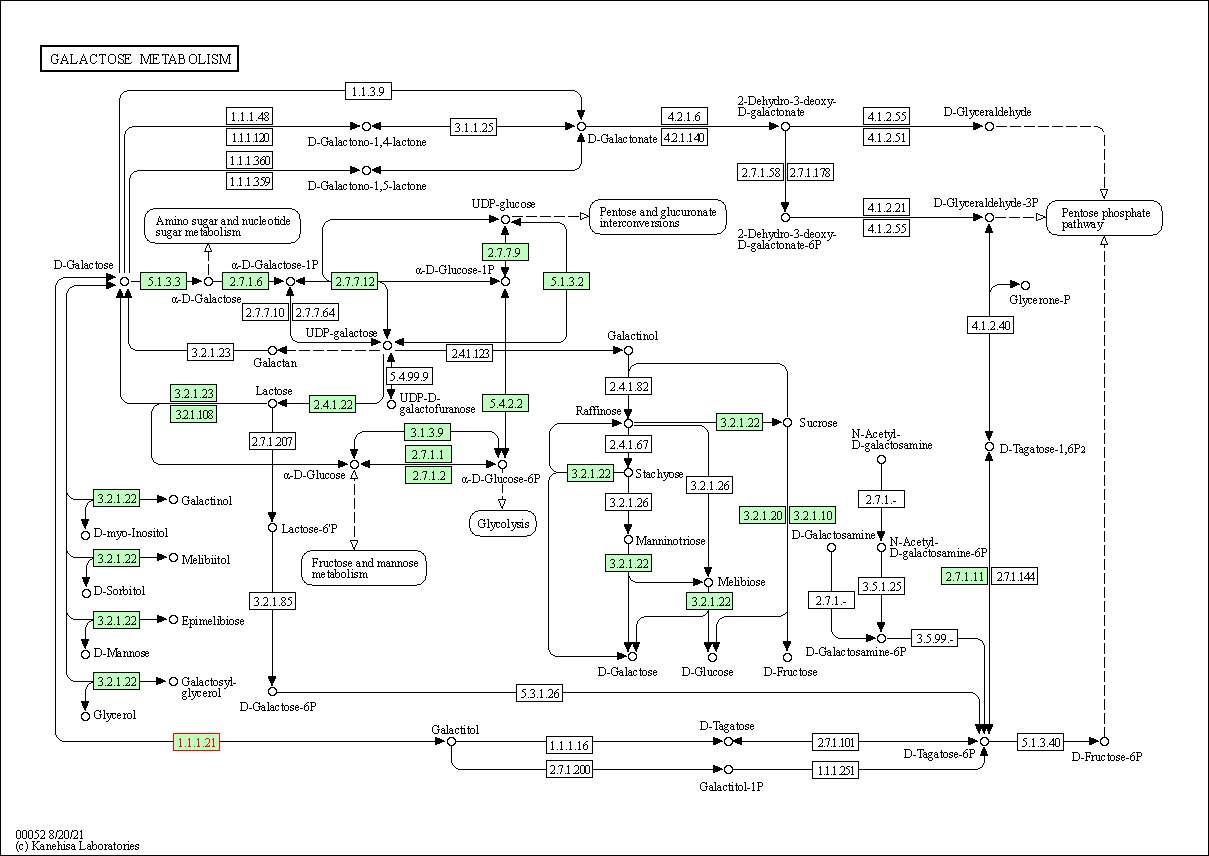
| Galactose metabolism | hsa00052 | Affiliated Target |

|
| Class: Metabolism => Carbohydrate metabolism | Pathway Hierarchy | ||
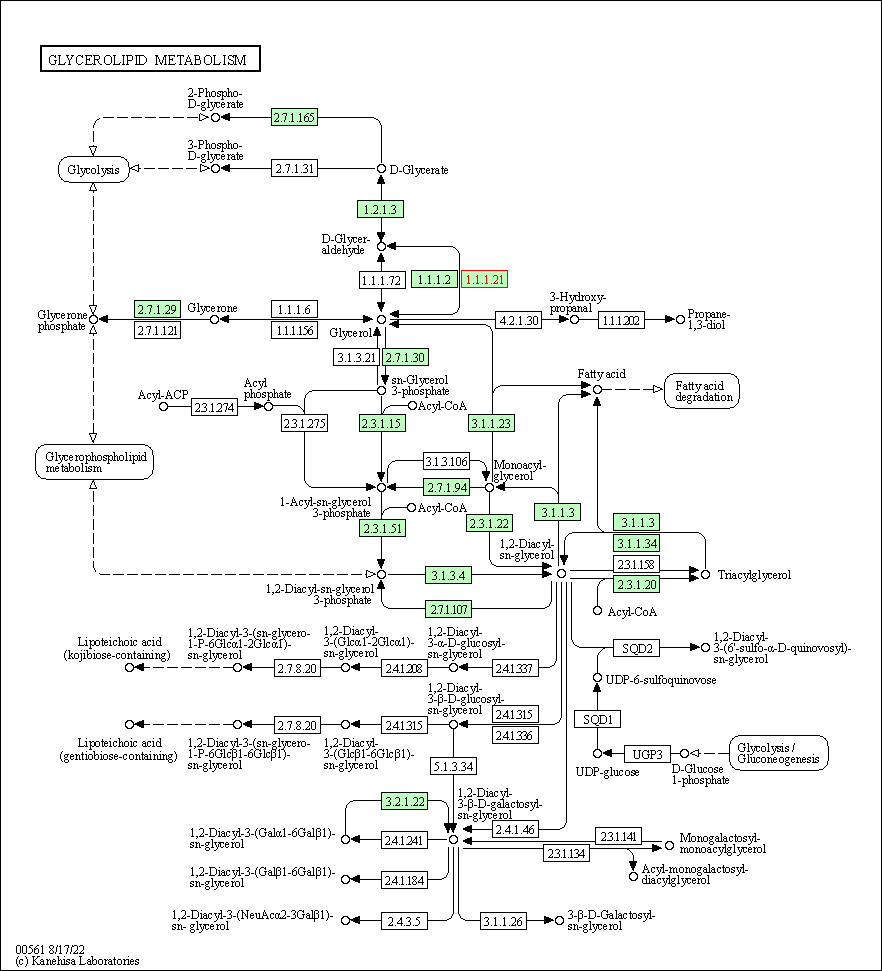
| Glycerolipid metabolism | hsa00561 | Affiliated Target |

|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Folate biosynthesis | hsa00790 | Affiliated Target |

|
| Class: Metabolism => Metabolism of cofactors and vitamins | Pathway Hierarchy | ||
| Degree | 5 | Degree centrality | 5.37E-04 | Betweenness centrality | 3.52E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.79E-01 | Radiality | 1.29E+01 | Clustering coefficient | 3.00E-01 |
| Neighborhood connectivity | 8.20E+00 | Topological coefficient | 2.44E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| BioCyc | [+] 2 BioCyc Pathways | + | ||||
| 1 | Methylglyoxal degradation III | |||||
| 2 | Acetone degradation I (to methylglyoxal) | |||||
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | Pentose and glucuronate interconversions | |||||
| 2 | Fructose and mannose metabolism | |||||
| 3 | Galactose metabolism | |||||
| 4 | Glycerolipid metabolism | |||||
| 5 | Metabolic pathways | |||||
| NetPath Pathway | [+] 2 NetPath Pathways | + | ||||
| 1 | IL1 Signaling Pathway | |||||
| 2 | TGF_beta_Receptor Signaling Pathway | |||||
| Pathwhiz Pathway | [+] 5 Pathwhiz Pathways | + | ||||
| 1 | Fructose and Mannose Degradation | |||||
| 2 | Pyruvate Metabolism | |||||
| 3 | Pterine Biosynthesis | |||||
| 4 | Glycerolipid Metabolism | |||||
| 5 | Galactose Metabolism | |||||
| WikiPathways | [+] 3 WikiPathways | + | ||||
| 1 | Metapathway biotransformation | |||||
| 2 | Polyol Pathway | |||||
| 3 | Metabolism of steroid hormones and vitamin D | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Inhibition of human lens aldose reductase by flavonoids, sulindac and indomethacin. Biochem Pharmacol. 1983 Jul 1;32(13):1995-8. | |||||
| REF 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5425). | |||||
| REF 4 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | |||||
| REF 5 | X-ray structure of the V301L aldo-keto reductase 1B10 complexed with NADP(+) and the potent aldose reductase inhibitor fidarestat: implications for inhibitor binding and selectivity. Chem Biol Interact. 2013 Feb 25;202(1-3):178-85. | |||||
| REF 6 | ClinicalTrials.gov (NCT00101426) Safety and Efficacy of AS-3201 in the Treatment of Diabetic Sensorimotor Polyneuropathy. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT02332005) 12-Month Efficacy and Safety of Diepalrestat in Adults With Diabetic Peripheral Neuropathy, a DB, Placebo-Controlled Study (DE-DPN). U.S. National Institutes of Health. | |||||
| REF 8 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 9 | ClinicalTrials.gov (NCT00252148) Safety and Immunogenicity of a Modified Vaccinia Ankara (MVA) HIV Vaccine in HIV Uninfected Adults. U.S. National Institutes of Health. | |||||
| REF 10 | Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B'/C candidate vaccine. PLoS One. 2010 Jan 25;5(1):e8816. | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7411). | |||||
| REF 12 | ClinicalTrials.gov (NCT00043797) Lidorestat (IDD 676) for the Treatment of Diabetic Neuropathy. U.S. National Institutes of Health. | |||||
| REF 13 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000031) | |||||
| REF 14 | ClinicalTrials.gov (NCT00568035) Safety and Efficacy Study of QR-333 in Patient's With Symptomatic Diabetic Neuropathy. U.S. National Institutes of Health. | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800029470) | |||||
| REF 16 | Metabolism of the aldose reductase inhibitor ALO1567 in man. Br J Clin Pharmacol. 1991 Aug;32(2):221-7. | |||||
| REF 17 | The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007 Oct;12(10):904-22. | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000020) | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003006) | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000017) | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001300) | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011583) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017463) | |||||
| REF 24 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7419). | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001173) | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800032059) | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004685) | |||||
| REF 28 | Studies on WF-3681, a novel aldose reductase inhibitor. IV. Effect of FR-62765, a derivative of WF-3681, on the diabetic neuropathy in rats. J Antibiot (Tokyo). 1991 Apr;44(4):441-4. | |||||
| REF 29 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7415). | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000008) | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002923) | |||||
| REF 32 | WF-2421, a new aldose reductase inhibitor produced from a fungus, Humicola grisea. J Antibiot (Tokyo). 1991 Feb;44(2):130-5. | |||||
| REF 33 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7418). | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000004) | |||||
| REF 35 | Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in Type 2 diabetic patients. J Diabetes Complications. 2001 Sep-Oct;15(5):241-4. | |||||
| REF 36 | Clinical investigation of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy in Japan: multicenter study. Diabetic Neuropathy Study Group in Japan. J Diabetes Complications. 1996 May-Jun;10(3):168-72. | |||||
| REF 37 | Diabetic complications in lens and nerve and their prevention by sulindac or sorbinil: two novel aldose reductase inhibitors. Invest Ophthalmol Vis Sci. 1983 Oct;24(10):1426-9. | |||||
| REF 38 | Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group study. Diabetes Care. 2001 Oct;24(10):1776-82. | |||||
| REF 39 | Aldose reductase inhibitor SNK-860. Nippon Rinsho. 1997 Nov;55 Suppl:212-5. | |||||
| REF 40 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2768). | |||||
| REF 41 | Effect of Polygonum hydropiper sulfated flavonoids on lens aldose reductase and related enzymes. J Nat Prod. 1996 Apr;59(4):443-5. | |||||
| REF 42 | Discovery of 3-[(4,5,7-trifluorobenzothiazol-2-yl)methyl]indole-N-acetic acid (lidorestat) and congeners as highly potent and selective inhibitors ... J Med Chem. 2005 May 5;48(9):3141-52. | |||||
| REF 43 | Effects of novel aldose reductase inhibitors, M16209 and M16287, on streptozotocin-induced diabetic neuropathy in rats. Eur J Pharmacol. 1991 Feb 7;193(2):185-91. | |||||
| REF 44 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 45 | A multicenter, double-blind, safety study of QR-333 for the treatment of symptomatic diabetic peripheral neuropathy. A preliminary report. J Diabetes Complications. 2005 Sep-Oct;19(5):247-53. | |||||
| REF 46 | Aldose reductase inhibitors: an update. Ann Pharmacother. 1993 Jun;27(6):751-4. | |||||
| REF 47 | Spiro[fluoreneisothiazolidin]one dioxides: new aldose reductase and L-hexonate dehydrogenase inhibitors. J Med Chem. 1991 Nov;34(11):3229-34. | |||||
| REF 48 | Minalrestat, an aldose reductase inhibitor, corrects the impaired microvascular reactivity in diabetes. J Pharmacol Exp Ther. 2003 Mar;304(3):1236-42. | |||||
| REF 49 | Ponalrestat, an aldose reductase inhibitor, inhibits cachexia syndrome induced by colon26 adenocarcinoma in mice. Anticancer Res. 1999 Sep-Oct;19(5B):4105-11. | |||||
| REF 50 | Studies on antidiabetic agents. IX. A new aldose reductase inhibitor, AD-5467, and related 1,4-benzoxazine and 1,4-benzothiazine derivatives: synthesis and biological activity. Chem Pharm Bull (Tokyo). 1990 May;38(5):1238-45. | |||||
| REF 51 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 52 | Genes in the Service of Therapeutic Index: Progress for Virus-Directed Enzyme Prodrug Therapy. JCO May 1, 2004 vol. 22 no. 9 1535-1537c. | |||||
| REF 53 | Comparison of the effects of Zopolrestat and Sorbinil on lens myo-inositol influx. Pharmacology. 1997 Feb;54(2):76-83. | |||||
| REF 54 | A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes. 2006 Oct;55(10):2757-62. | |||||
| REF 55 | Aldose reductase inhibitors and prevention of galactose cataracts in rats. Invest Ophthalmol Vis Sci. 1989 Jul;30(7):1623-32. | |||||
| REF 56 | A controlled trial of sorbinil, an aldose reductase inhibitor, in chronic painful diabetic neuropathy. Diabetes. 1983 Oct;32(10):938-42. | |||||
| REF 57 | Recent clinical experience with aldose reductase inhibitors. J Diabetes Complications. 1992 Jan-Mar;6(1):39-44. | |||||
| REF 58 | Pharmacological profiles of a novel aldose reductase inhibitor, SPR-210, and its effects on streptozotocin-induced diabetic rats. Jpn J Pharmacol. 1994 Feb;64(2):115-24. | |||||
| REF 59 | The effects of zenarestat, an aldose reductase inhibitor, on minimal F-wave latency and nerve blood flow in streptozotocin-induced diabetic rats. Life Sci. 2001 Feb 9;68(12):1439-48. | |||||
| REF 60 | Synthesis and aldose reductase inhibitory activity of substituted 2-oxoquinoline-1-acetic acid derivatives. J Med Chem. 1986 Oct;29(10):2024-8. | |||||
| REF 61 | Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proc Natl Acad Sci U S A. 2007 Dec 26;104(52):20764-9. | |||||
| REF 62 | 1-Benzopyran-4-one antioxidants as aldose reductase inhibitors. J Med Chem. 1999 Jun 3;42(11):1881-93. | |||||
| REF 63 | Design and synthesis of novel series of pyrrole based chemotypes and their evaluation as selective aldose reductase inhibitors. A case of bioisoste... Bioorg Med Chem. 2010 Mar 15;18(6):2107-2114. | |||||
| REF 64 | Design and synthesis of N-(3,5-difluoro-4-hydroxyphenyl)benzenesulfonamides as aldose reductase inhibitors. Bioorg Med Chem. 2008 Apr 1;16(7):3926-32. | |||||
| REF 65 | A diverse series of substituted benzenesulfonamides as aldose reductase inhibitors with antioxidant activity: design, synthesis, and in vitro activ... J Med Chem. 2010 Nov 11;53(21):7756-66. | |||||
| REF 66 | Correlation of binding constants and molecular modelling of inhibitors in the active sites of aldose reductase and aldehyde reductase. Bioorg Med Chem. 2009 Feb 1;17(3):1244-50. | |||||
| REF 67 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 68 | Isoxazolidine-3,5-diones as lens aldose reductase inhibitors. J Med Chem. 1982 Jun;25(6):745-7. | |||||
| REF 69 | A novel series of non-carboxylic acid, non-hydantoin inhibitors of aldose reductase with potent oral activity in diabetic rat models: 6-(5-chloro-3... J Med Chem. 2005 Oct 6;48(20):6326-39. | |||||
| REF 70 | Flavonoids with anti-cataract activity from Brickellia arguta. J Nat Prod. 1984 Mar-Apr;47(2):316-9. | |||||
| REF 71 | 6,7-Dihydroxy-4-phenylcoumarin as inhibitor of aldose reductase 2. Bioorg Med Chem Lett. 2010 Oct 1;20(19):5630-3. | |||||
| REF 72 | The Effect of Halogen-to-Hydrogen Bond Substitution on Human Aldose Reductase Inhibition. ACS Chem Biol. 2015 Jul 17;10(7):1637-42. | |||||
| REF 73 | Erigeroflavanone, a flavanone derivative from the flowers of Erigeron annuus with protein glycation and aldose reductase inhibitory activity. J Nat Prod. 2008 Apr;71(4):713-5. | |||||
| REF 74 | Synthesis, characterization and vasculoprotective effects of nitric oxide-donating derivatives of chrysin. Bioorg Med Chem. 2010 May 1;18(9):3020-5. | |||||
| REF 75 | New flavonol oligoglycosides and polyacylated sucroses with inhibitory effects on aldose reductase and platelet aggregation from the flowers of Pru... J Nat Prod. 2002 Aug;65(8):1151-5. | |||||
| REF 76 | Structures of new friedelane-type triterpenes and eudesmane-type sesquiterpene and aldose reductase inhibitors from Salacia chinensis. J Nat Prod. 2003 Sep;66(9):1191-6. | |||||
| REF 77 | Inhibitors of aldose reductase and formation of advanced glycation end-products in moutan cortex (Paeonia suffruticosa). J Nat Prod. 2009 Aug;72(8):1465-70. | |||||
| REF 78 | The molecular basis for inhibition of sulindac and its metabolites towards human aldose reductase. FEBS Lett. 2012 Jan 2;586(1):55-9. | |||||
| REF 79 | Partial inhibition of aldose reductase by nitazoxanide and its molecular basis. ChemMedChem. 2012 Nov;7(11):1921-3. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

