Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0J6JZ
|
|||
| Former ID |
DNC008399
|
|||
| Drug Name |
MINALRESTAT
|
|||
| Synonyms |
Minalrestat; WAY-ARI-509; 129688-50-2; Minalrestat [USAN:INN]; Spiro(isoquinoline-4(1H),3'-pyrrolidine)-1,2',3,5'(2H)-tetrone, 2-((4-bromo-2-fluorophenyl)-methyl)-6-fluoro-;; 2-(4-Bromo-2-fluorobenzyl)-6-fluorospiro(isoquinoline-4(1H),3'-pyrrolidine)-1,2',3,5'(2H)-tetrone; Spiro(isoquinoline-4(1H),3'-pyrrolidine)-1,2',3,5'(2H)-tetrone, 2-((4-Bromo-2-fluorophenyl)methyl)-6-fluoro-; Bffipt; Minalrestat (2); AC1L4RBZ; Minalrestat (USAN/INN); SCHEMBL49649; CHEMBL273910; BDBM228819; AKOS022180622; SB19712; API0013644
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Diabetic complication [ICD-11: 5A2Y; ICD-9: 253.5, 588.1] | Discontinued in Phase 3 | [1] | |
| Structure |
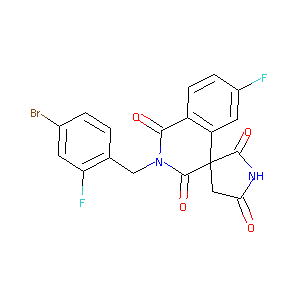 |
Download2D MOL |
||
| Formula |
C19H11BrF2N2O4
|
|||
| Canonical SMILES |
C1C(=O)NC(=O)C12C3=C(C=CC(=C3)F)C(=O)N(C2=O)CC4=C(C=C(C=C4)Br)F
|
|||
| InChI |
1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)
|
|||
| InChIKey |
BMHZAHGTGIZZCT-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 129688-50-2
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aldose reductase (AKR1B1) | Target Info | Modulator | [2] |
| BioCyc | Methylglyoxal degradation III | |||
| Acetone degradation I (to methylglyoxal) | ||||
| KEGG Pathway | Pentose and glucuronate interconversions | |||
| Fructose and mannose metabolism | ||||
| Galactose metabolism | ||||
| Glycerolipid metabolism | ||||
| Metabolic pathways | ||||
| NetPath Pathway | IL1 Signaling Pathway | |||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Fructose and Mannose Degradation | |||
| Pyruvate Metabolism | ||||
| Pterine Biosynthesis | ||||
| Glycerolipid Metabolism | ||||
| Galactose Metabolism | ||||
| WikiPathways | Metapathway biotransformation | |||
| Polyol Pathway | ||||
| Metabolism of steroid hormones and vitamin D | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003006) | |||
| REF 2 | Minalrestat, an aldose reductase inhibitor, corrects the impaired microvascular reactivity in diabetes. J Pharmacol Exp Ther. 2003 Mar;304(3):1236-42. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

