Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D4PL6G
|
|||
| Drug Name |
BNV-222
|
|||
| Synonyms |
Diepalrestat choline; UNII-16AHE7410O; 16AHE7410O; CHEMBL4297277; Q27251797; 1665300-21-9; Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, (5Z)-5-((2E)-2-methyl-3-phenyl-2-propen-1-ylidene)-4-oxo-2-thioxo-3-thiazolidineacetate, (5Z)-5-((2E)-2-methyl-3-phenyl-2-propen-1-ylidene)-4-oxo-2-thioxo-3-thiazolidineacetate (1:1:1)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Diabetic neuropathy [ICD-11: 8C0Z] | Phase 2/3 | [1] | |
| Company |
BioNevia Pharmaceuticals
|
|||
| Structure |
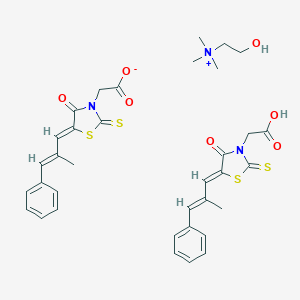 |
Download2D MOL
|
||
| Formula |
C35H39N3O7S4
|
|||
| Canonical SMILES |
CC(=CC1=CC=CC=C1)C=C2C(=O)N(C(=S)S2)CC(=O)O.CC(=CC1=CC=CC=C1)C=C2C(=O)N(C(=S)S2)CC(=O)[O-].C[N+](C)(C)CCO
|
|||
| InChI |
1S/2C15H13NO3S2.C5H14NO/c2*1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12;1-6(2,3)4-5-7/h2*2-8H,9H2,1H3,(H,17,18);7H,4-5H2,1-3H3/q;;+1/p-1/b2*10-7+,12-8-;
|
|||
| InChIKey |
WKSNHZZSMLFYFI-UROVXFGGSA-M
|
|||
| CAS Number |
CAS 1665300-21-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aldose reductase (AKR1B1) | Target Info | Inhibitor | [1] |
| BioCyc | Methylglyoxal degradation III | |||
| Acetone degradation I (to methylglyoxal) | ||||
| KEGG Pathway | Pentose and glucuronate interconversions | |||
| Fructose and mannose metabolism | ||||
| Galactose metabolism | ||||
| Glycerolipid metabolism | ||||
| Metabolic pathways | ||||
| NetPath Pathway | IL1 Signaling Pathway | |||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Fructose and Mannose Degradation | |||
| Pyruvate Metabolism | ||||
| Pterine Biosynthesis | ||||
| Glycerolipid Metabolism | ||||
| Galactose Metabolism | ||||
| WikiPathways | Metapathway biotransformation | |||
| Polyol Pathway | ||||
| Metabolism of steroid hormones and vitamin D | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02332005) 12-Month Efficacy and Safety of Diepalrestat in Adults With Diabetic Peripheral Neuropathy, a DB, Placebo-Controlled Study (DE-DPN). U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

