Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0U9TM
|
|||
| Former ID |
DNC001141
|
|||
| Drug Name |
Ponalrestat
|
|||
| Synonyms |
PONALRESTAT; statil; 72702-95-5; Ponalrestatum [Latin]; UNII-2CV0A5G64E; ICI-128436; ICI 128,436; CHEMBL7679; C17H12BrFN2O3; 2CV0A5G64E; 2-(3-(4-bromo-2-fluorobenzyl)-4-oxo-3,4-dihydrophthalazin-1-yl)acetic acid; NCGC00024824-01; 3-(4-Bromo-2-fluorobenzyl)-3,4-dihydro-4-oxo-1-phthalazineacetic acid; 1-Phthalazineaceticacid, 3-[(4-bromo-2-fluorophenyl)methyl]-3,4-dihydro-4-oxo-; Ponalrestatum; 1-Phthalazineacetic acid, 3-((4-bromo-2-fluorophenyl)methyl)-3,4-dihydro-4-oxo-; Prodiax; Ponalrestat [USAN:BAN:INN]; ISIS-5GLT2rx
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Gout [ICD-11: FA25; ICD-10: M10] | Discontinued in Phase 3 | [1] | |
| Diabetic complication [ICD-11: 5A2Y; ICD-9: 253.5, 588.1] | Investigative | [2] | ||
| Structure |
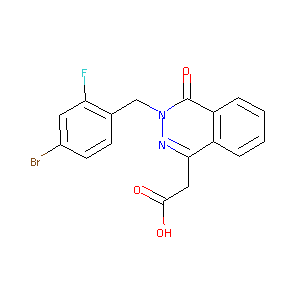 |
Download2D MOL |
||
| Formula |
C17H12BrFN2O3
|
|||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=NN(C2=O)CC3=C(C=C(C=C3)Br)F)CC(=O)O
|
|||
| InChI |
1S/C17H12BrFN2O3/c18-11-6-5-10(14(19)7-11)9-21-17(24)13-4-2-1-3-12(13)15(20-21)8-16(22)23/h1-7H,8-9H2,(H,22,23)
|
|||
| InChIKey |
LKBFFDOJUKLQNY-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 72702-95-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
5616278, 8153244, 11113738, 11120263, 11120751, 11121239, 11342114, 11362297, 11364377, 11366939, 11369501, 11371896, 11374993, 11377663, 11485129, 11487699, 11489283, 11490863, 11493002, 11495297, 12013115, 14829960, 17397894, 26680549, 29224333, 47364952, 50065127, 53530998, 57322692, 85789490, 85789665, 103123627, 103165638, 103922759, 104308777, 117389119, 124637034, 124886948, 128500409, 134339117, 134340376, 135011929, 135698020, 135727686, 137122051, 139060527, 142773112, 144204481, 162022632, 162655628
|
|||
| ChEBI ID |
CHEBI:93199
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aldose reductase (AKR1B1) | Target Info | Inhibitor | [3] |
| Sodium/glucose cotransporter 2 (SLC5A4) | Target Info | Blocker | [2] | |
| BioCyc | Methylglyoxal degradation III | |||
| Acetone degradation I (to methylglyoxal) | ||||
| KEGG Pathway | Pentose and glucuronate interconversions | |||
| Fructose and mannose metabolism | ||||
| Galactose metabolism | ||||
| Glycerolipid metabolism | ||||
| Metabolic pathways | ||||
| NetPath Pathway | IL1 Signaling Pathway | |||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Fructose and Mannose Degradation | |||
| Pyruvate Metabolism | ||||
| Pterine Biosynthesis | ||||
| Glycerolipid Metabolism | ||||
| Galactose Metabolism | ||||
| Reactome | Hexose transport | |||
| Na+-dependent glucose transporters | ||||
| Inositol transporters | ||||
| WikiPathways | NRF2 pathway | |||
| Metapathway biotransformation | ||||
| Polyol Pathway | ||||
| Metabolism of steroid hormones and vitamin D | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000017) | |||
| REF 2 | Clinical pipeline report, company report or official report of ISIS Pharmaceuticals (2009). | |||
| REF 3 | Ponalrestat, an aldose reductase inhibitor, inhibits cachexia syndrome induced by colon26 adenocarcinoma in mice. Anticancer Res. 1999 Sep-Oct;19(5B):4105-11. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

