Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0BH6B
|
|||
| Former ID |
DNC013662
|
|||
| Drug Name |
Apigenin-7-O-beta-D-glucuronide methyl ester
|
|||
| Synonyms |
53538-13-9; (2S,3S,4S,5R,6S)-Methyl 3,4,5-trihydroxy-6-((5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)tetrahydro-2H-pyran-2-carboxylate; CHEMBL464868; Apigenin 7-O-methylglucuronide; apigenin-7-O-beta-D-glucuronide methyl ester; MolPort-035-684-098; Apigenin 7-(6-O-methylglucuronide); ZINC40874044; BDBM50251270; AKOS024258585; AJ-103820; ST24034139
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
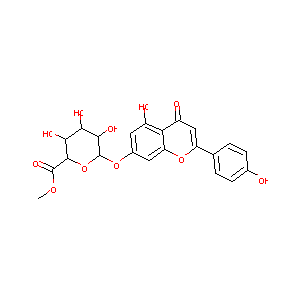 |
Download2D MOL |
||
| Formula |
C22H20O11
|
|||
| Canonical SMILES |
COC(=O)C1C(C(C(C(O1)OC2=CC(=C3C(=C2)OC(=CC3=O)C4=CC=C(C=C4)O)O)O)O)O
|
|||
| InChI |
1S/C22H20O11/c1-30-21(29)20-18(27)17(26)19(28)22(33-20)31-11-6-12(24)16-13(25)8-14(32-15(16)7-11)9-2-4-10(23)5-3-9/h2-8,17-20,22-24,26-28H,1H3/t17-,18-,19+,20-,22+/m0/s1
|
|||
| InChIKey |
XXKIWCKZQFBXIR-SXFAUFNYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aldose reductase (AKR1B1) | Target Info | Inhibitor | [1] |
| BioCyc | Methylglyoxal degradation III | |||
| Acetone degradation I (to methylglyoxal) | ||||
| KEGG Pathway | Pentose and glucuronate interconversions | |||
| Fructose and mannose metabolism | ||||
| Galactose metabolism | ||||
| Glycerolipid metabolism | ||||
| Metabolic pathways | ||||
| NetPath Pathway | IL1 Signaling Pathway | |||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Fructose and Mannose Degradation | |||
| Pyruvate Metabolism | ||||
| Pterine Biosynthesis | ||||
| Glycerolipid Metabolism | ||||
| Galactose Metabolism | ||||
| WikiPathways | Metapathway biotransformation | |||
| Polyol Pathway | ||||
| Metabolism of steroid hormones and vitamin D | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Erigeroflavanone, a flavanone derivative from the flowers of Erigeron annuus with protein glycation and aldose reductase inhibitory activity. J Nat Prod. 2008 Apr;71(4):713-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

