Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0C4NY
|
|||
| Former ID |
DIB013211
|
|||
| Drug Name |
Promestriene
|
|||
| Synonyms |
BCP9000134; VA11593; A824465; I06-0300
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acne vulgaris [ICD-11: ED80; ICD-10: L70.0; ICD-9: 706.1] | Approved | [1] | |
| Structure |
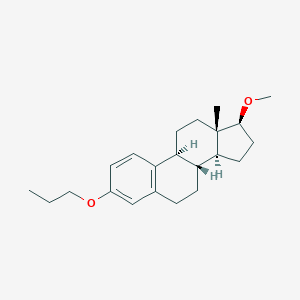 |
Download2D MOL |
||
| Formula |
C22H32O2
|
|||
| Canonical SMILES |
CCCOC1=CC2=C(C=C1)C3CCC4(C(C3CC2)CCC4OC)C
|
|||
| InChI |
1S/C22H32O2/c1-4-13-24-16-6-8-17-15(14-16)5-7-19-18(17)11-12-22(2)20(19)9-10-21(22)23-3/h6,8,14,18-21H,4-5,7,9-13H2,1-3H3/t18-,19-,20+,21+,22+/m1/s1
|
|||
| InChIKey |
IUWKNLFTJBHTSD-AANPDWTMSA-N
|
|||
| CAS Number |
CAS 39219-28-8
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:135402
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Promestriene, a specific topic estrogen. Review of 40 years of vaginal atrophy treatment: is it safe even in cancer patients. Anticancer Drugs. 2013 Nov;24(10):989-98. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

