Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E1XL
|
|||
| Former ID |
DAP000502
|
|||
| Drug Name |
Levetiracetam
|
|||
| Synonyms |
102767-28-2; Keppra; (S)-2-(2-Oxopyrrolidin-1-yl)butanamide; Keppra XR; Levetiracetamum; ucb L059; (2S)-2-(2-oxopyrrolidin-1-yl)butanamide; UCB-L 059; UCB-L059; Spritam; (S)-alpha-Ethyl-2-oxo-1-pyrrolidineacetamide; (-)-(S)-alpha-Ethyl-2-oxo-1-pyrrolidineacetamide; SIB-S1; UNII-44YRR34555; 1-Pyrrolidineacetamide, alpha-ethyl-2-oxo-, (alphaS)-; UCB-22059; Levetiracetamum [INN-Latin]; Levetiractam; CHEBI:6437; ucb L060; Levetiracetam In Sodium Chloride; 44YRR34555; Levroxa; 1-Pyrrolidineacetamide, alpha-ethyl-2-oxo-,; Keppra; Leviteracetam; Torleva; Levetiracetam [INN]; Ucb L060; Etiracetam levo-isomer; Keppra (TN); L-059; Etiracetam, S-isomer; Keppra, Keppra XR),Levetiracetam; Levetriacetam
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Epilepsy [ICD-11: 8A60-8A68] | Approved | [1], [2] | |
| Fibromyalgia [ICD-11: MG30.01; ICD-10: M79.7; ICD-9: 729.1] | Approved | [3] | ||
| Therapeutic Class |
Anticonvulsants
|
|||
| Company |
UCB Pharmaceuticals Inc
|
|||
| Structure |
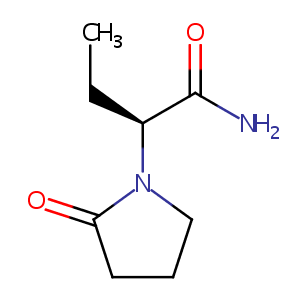 |
Download2D MOL |
||
| Formula |
C8H14N2O2
|
|||
| Canonical SMILES |
CCC(C(=O)N)N1CCCC1=O
|
|||
| InChI |
1S/C8H14N2O2/c1-2-6(8(9)12)10-5-3-4-7(10)11/h6H,2-5H2,1H3,(H2,9,12)/t6-/m0/s1
|
|||
| InChIKey |
HPHUVLMMVZITSG-LURJTMIESA-N
|
|||
| CAS Number |
CAS 102767-28-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10043, 7847774, 7979767, 11039358, 11528730, 12013706, 15321884, 15321885, 26719810, 39317891, 46386557, 48416166, 49681695, 49830155, 50363327, 56310923, 56312936, 56312991, 56313270, 57359126, 68530790, 90341134, 91614180, 92308297, 92308916, 92713787, 93166400, 99437106, 103397964, 113863233, 118048597, 118317474, 121361595, 124658926, 124757185, 124801236, 125163989, 125307963, 126629740, 126645371, 126655872, 128093760, 131293132, 134338679, 135017078, 135610154, 135684175, 135692116, 135697608, 136375572
|
|||
| ChEBI ID |
CHEBI:6437
|
|||
| ADReCS Drug ID | BADD_D01265 | |||
| SuperDrug ATC ID |
N03AX14
|
|||
| SuperDrug CAS ID |
cas=102767282
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 3A receptor (HTR3A) | Target Info | Antagonist | [3] |
| Synaptic vesicle glycoprotein 2A (SV2A) | Target Info | Modulator | [4] | |
| KEGG Pathway | Serotonergic synapse | |||
| ECM-receptor interaction | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | 5HT3 type receptor mediated signaling pathway | |||
| Reactome | Ligand-gated ion channel transport | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Iron uptake and transport | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6826). | |||
| REF 2 | Use of second-generation antiepileptic drugs in the pediatric population. Paediatr Drugs. 2008;10(4):217-54. | |||
| REF 3 | Emerging therapies for fibromyalgia. Expert Opin Emerg Drugs. 2008 Mar;13(1):53-62. | |||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

