Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M0QX
|
|||
| Former ID |
DCL000307
|
|||
| Drug Name |
MK-591
|
|||
| Synonyms |
Quiflapon; Quiflapon [INN]; L 686708; MK 0591; MK 591; L-686708; MK-0591; L-686,708; 3-(1-(4-chlorobenzyl-3-(t-butylthio)-5-(quinolin-2-ylmethoxy)indol-2-yl))-2,2-dimethyl propanoic acid; 3-(tert-Butylthio)-1-(p-chlorobenzyl)-alpha,alpha-dimethyl-5-(2-quinolylmethoxy)indole-2-propionic acid; 3-(tert-butylthio)-1-(p-chlorobenzyl)-a,a-dimethyl-5-(2-quinolylmethoxy)indole-2-propionic acid; 3-[3-tert-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8; ICD-9: 493] | Discontinued in Phase 2 | [1] | |
| Company |
Merck
|
|||
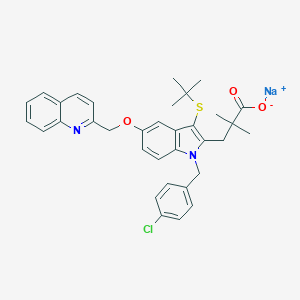
| Structure |
 |
Download2D MOL
|
||
| Formula |
C34H34ClN2NaO3S
|
|||
| Canonical SMILES |
CC(C)(C)SC1=C(N(C2=C1C=C(C=C2)OCC3=NC4=CC=CC=C4C=C3)CC5=CC=C(C=C5)Cl)CC(C)(C)C(=O)[O-].[Na+]
|
|||
| InChI |
1S/C34H35ClN2O3S.Na/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39;/h6-18H,19-21H2,1-5H3,(H,38,39);/q;+1/p-1
|
|||
| InChIKey |
YPURUCMVRRNPHJ-UHFFFAOYSA-M
|
|||
| CAS Number |
CAS 147030-01-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 5-lipoxygenase (5-LOX) | Target Info | Inhibitor | [2], [3] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Vitamin D Receptor Pathway | |||
| Arachidonic acid metabolism | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001712) | |||
| REF 2 | Antileukotriene therapy for asthma. Am J Health Syst Pharm. 1996 Dec 1;53(23):2821-30; quiz 2877-8. | |||
| REF 3 | Inhibition of antigen-induced contraction of guinea-pig airways by a leukotriene synthesis inhibitor, BAY x1005. Eur J Pharmacol. 1994 Jun 2;258(1-2):95-102. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

