Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0O6GC
|
|||
| Former ID |
DAP000366
|
|||
| Drug Name |
Methysergide
|
|||
| Synonyms |
Deseril; Desernil; Desernyl; Deseryl; Desril; Dimethylergometrin; Methylmethylergonovine; Methysergid; Methysergidum; Metisergide; Metisergido; Sansert; Methyllysergic acid butanolamide; Metisergide [DCIT]; UML 491; Deseril (TN); Methysergidum [INN-Latin]; Metisergido [INN-Spanish]; Sansert (TN); UML-491; Methysergide (USAN/INN); Methysergide [USAN:INN:BAN]; N-(alpha-(Hydroxymethyl)propyl)-1-methyl-dextro-lysergamide; N-(1-(Hydroxymethyl)propyl)-1-methyl-dextro-(+)-lysergamide; (+)-9,10-Didehydro-N-(1-(hydroxymethyl)propyl)-1,6-dimethylergoline-8beta-carboxamide; (+)-N-(1-(Hydroxymethyl)propyl)-1-methyl-D-lysergamide; (8beta)-N-[(1S)-1-(hydroxymethyl)propyl]-1,6-dimethyl-9,10-didehydroergoline-8-carboxamide; 1-Methyl-D-lysergic acid butanolamide; 1-Methyl-dextro-lysergic acid (+)-1-hydroxy-2-butylamide; 1-Methylmethylergonovine; 9,10-Didehydro-N-(1-(hydroxymethyl)propyl)-1,6-dimethylergoline-8-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Migraine [ICD-11: 8A80; ICD-10: G43, G43.9; ICD-9: 346] | Approved | [1], [2] | |
| Therapeutic Class |
Vasoconstrictor Agents
|
|||
| Company |
Norvatis Phamaceuticals Corporation
|
|||
| Structure |
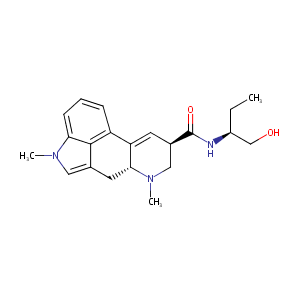 |
Download2D MOL |
||
| Formula |
C21H27N3O2
|
|||
| Canonical SMILES |
CCC(CO)NC(=O)C1CN(C2CC3=CN(C4=CC=CC(=C34)C2=C1)C)C
|
|||
| InChI |
1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1
|
|||
| InChIKey |
KPJZHOPZRAFDTN-ZRGWGRIASA-N
|
|||
| CAS Number |
CAS 361-37-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9408, 7849416, 7979958, 8156955, 26751590, 29228253, 47365379, 47515489, 48334682, 48416266, 49965410, 50104228, 50428725, 53790023, 57325757, 85787459, 90340563, 92309300, 103292415, 103940495, 104321196, 124750057, 124886858, 124886859, 128415730, 134337676, 134973635, 135650596, 137002477, 144204446, 160963595, 170464684, 175266387, 176484551, 179236187, 221673418, 226426779, 252614966
|
|||
| ChEBI ID |
CHEBI:92629
|
|||
| ADReCS Drug ID | BADD_D01439 ; BADD_D01440 | |||
| SuperDrug ATC ID |
N02CA04
|
|||
| SuperDrug CAS ID |
cas=000361375
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Dorea formicigenerans ATCC 27755
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Methysergide maleate can be metabolized by Dorea formicigenerans ATCC 27755 (log2FC = -0.374; p = 0.014). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Victivallales | ||||
|
Studied Microbe: Victivallis vadensis ATCC BAA-548
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Methysergide maleate can be metabolized by Victivallis vadensis ATCC BAA-548 (log2FC = -0.348; p = 0.034). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 2C receptor (HTR2C) | Target Info | Antagonist | [4], [5], [6], [7] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Gap junction | ||||
| Serotonergic synapse | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | 5HT2 type receptor mediated signaling pathway | |||
| Reactome | Serotonin receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Serotonin Receptor 2 and ELK-SRF/GATA4 signaling | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 134). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 012516. | |||
| REF 3 | Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019 Jun;570(7762):462-467. | |||
| REF 4 | Spinal serotonin receptor activation modulates the exercise ventilatory response with increased dead space in goats. Respir Physiol Neurobiol. 2008 May 31;161(3):230-8. | |||
| REF 5 | Intake of fermented soybean (natto) increased locomotor activity in mice. Biol Pharm Bull. 2007 Apr;30(4):845-6. | |||
| REF 6 | Serotonergic mechanisms of the lateral parabrachial nucleus in renal and hormonal responses to isotonic blood volume expansion. Am J Physiol Regul Integr Comp Physiol. 2007 Mar;292(3):R1190-7. | |||
| REF 7 | p-Chloroamphetamine, a serotonin-releasing drug, elicited in rats a hyperglycemia mediated by the 5-HT1A and 5-HT2B/2C receptors. Eur J Pharmacol. 1998 Oct 23;359(2-3):185-90. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

