Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0O6IU
|
|||
| Former ID |
DAP000224
|
|||
| Drug Name |
Phenylephrine
|
|||
| Synonyms |
Cyclomydril; Dilatair; Dionephrine; Doktors; Duration; Fenilefrina; Isophrim; Isophrin; Mesaton; Mesatone; Mesatonum; Metaoxedrin; Metaoxedrine; Metaoxedrinum; Metasympatol; Metasynephrine; Metsatonum; Mezaton; Mydfrin; Neofrin; Neosynephrine; Nostril; Ocugestrin; Phenoptic; Phenylephrinum; Spersaphrine; Visadron; Alcon Efrin; Isopto Frin; Minims Phenylephrine; Nostril Spray Pump; Nostril Spray Pump Mild; Phenylephrine Minims; Prefrin Liquifilm; Relief Eye Drops for Red Eyes; Alconefrin Nasal Drops 12; Alconefrin Nasal Drops 25; Alconefrin Nasal Drops 50; Alconefrin Nasal Spray 25; Ah-Chew; Ak-dilate; Ak-nefrin; Fenilefrina [INN-Spanish]; I-Phrine; L-Phenylephedrine; L-Phenylephrine; M-Methylaminoethanolphenol; M-Oxedrine; M-Sympathol; M-Sympatol; M-Synephrine; Mydfrin (TN); Neo-Synephrine; Neo-Synephrine Nasal Drops; Neo-Synephrine Nasal Jelly; Neo-Synephrine Nasal Spray; Ocu-Phrin Sterile Eye Drops; Phenylephrine (INN); Phenylephrine Minims (TN); Phenylephrine [INN:BAN]; Phenylephrinum [INN-Latin]; R(-)-Phenylephrine; L-(3-Hydroxyphenyl)-N-methylethanolamine; L-1-(m-Hydroxyphenyl)-2-methylaminoethanol; L-m-Hydroxy-alpha-((methylamino)methyl)benzyl alcohol; L-alpha-Hydroxy-beta-methylamino-3-hydroxy-L-ethylbenzene; Tannins, compds. with (R)-3-hydroxy-alpha-((methylamino)methyl)benzenemethanol; Benzenemethanol, 3-hydroxy-alpha-((methylamino)methyl)-, (R)-(9CI); (-)-m-Hydroxy-alpha-(methylaminomethyl)benzyl alcohol; (R)-2-Hydroxy-2-(3-hydroxyphenyl)-N-methylethylamine; (R)-3-Hydroxy-alpha-((methylamino)methyl)benzenemethanol; 3-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Fecal incontinence [ICD-11: ME07; ICD-10: R15] | Approved | [1] | |
| Ophthalmic graves disease [ICD-11: 5A02.0; ICD-10: E05.0, H06.2] | Approved | [2], [3] | ||
| Therapeutic Class |
Ophthalmologicals
|
|||
| Company |
Alcon Canada Inc
|
|||
| Structure |
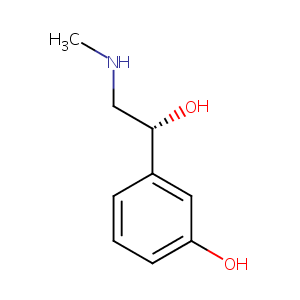 |
Download2D MOL |
||
| Formula |
C9H13NO2
|
|||
| Canonical SMILES |
CNCC(C1=CC(=CC=C1)O)O
|
|||
| InChI |
1S/C9H13NO2/c1-10-6-9(12)7-3-2-4-8(11)5-7/h2-5,9-12H,6H2,1H3/t9-/m0/s1
|
|||
| InChIKey |
SONNWYBIRXJNDC-VIFPVBQESA-N
|
|||
| CAS Number |
CAS 61-76-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9645, 598369, 3138730, 7980308, 8153766, 11111644, 11335772, 11361011, 11363995, 11366557, 11369119, 11371740, 11374452, 11377281, 11461983, 11485016, 11489096, 11490586, 11492782, 11494915, 11533358, 15147117, 15219459, 29225054, 46506961, 47216760, 47440232, 47588980, 47885392, 48184976, 48334474, 48416424, 49892965, 50100321, 50104255, 50104256, 53787092, 56311763, 56311811, 77849473, 87574602, 90340906, 93166315, 96025051, 103367925, 104310950, 117480201, 124750098, 126524414, 126687263
|
|||
| ChEBI ID |
CHEBI:8093
|
|||
| ADReCS Drug ID | BADD_D01762 ; BADD_D01763 | |||
| SuperDrug ATC ID |
C01CA06; R01AA04; R01AB01; R01BA03; S01FB01; S01GA05
|
|||
| SuperDrug CAS ID |
cas=000059427
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019494) | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 485). | |||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040654. | |||
| REF 4 | MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res. 2010 Apr;159(2):755-64. | |||
| REF 5 | Intracellular Ca2+ and adrenergic responsiveness of cardiac myocytes in streptozotocin-induced diabetes. Clin Exp Pharmacol Physiol. 1999 Apr;26(4):347-53. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

