Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DBND71
|
|||
| Drug Name |
GS-9674
|
|||
| Synonyms |
Cilofexor; 1418274-28-8; UNII-YUN2306954; PX104; 1418274-28-8 (free acid); YUN2306954; 2-(3-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)phenyl)-3-hydroxyazetidin-1-yl)isonicotinic acid; GS 9674; 2-[3-[2-chloro-4-[[5-cyclopropyl-3-(2,6-dichlorophenyl)-1,2-oxazol-4-yl]methoxy]phenyl]-3-hydroxyazetidin-1-yl]pyridine-4-carboxylic acid; Cilofexor [INN]; Cilofexor (GS(c)\\9674); CHEMBL4297613; SCHEMBL14641986; GTPL10644; BCP29283; BDBM50511109; GS9674; s6547; DB15168; example 13/9 [US10485795B2]; HY-109083; CS-0039260; 2-(3-(2-Chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-4-isoxazolyl)methoxy)phenyl)-3-hydroxy-1-azetidinyl)-4-pyridinecarboxylic acid; 4-Pyridinecarboxylic acid, 2-(3-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-4-isoxazolyl)methoxy)phenyl)-3-hydroxy-1-azetidinyl)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Primary sclerosing cholangitis [ICD-11: DB96.2; ICD-10: K83.0] | Phase 3 | [1] | |
| Non-alcoholic steatohepatitis [ICD-11: DB92.1; ICD-10: K75.8; ICD-9: 571.8] | Phase 2 | [2] | ||
| Structure |
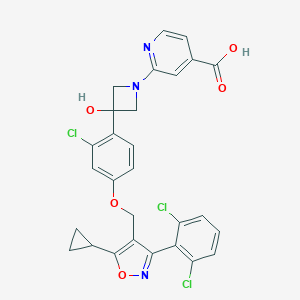 |
Download2D MOL |
||
| Formula |
C28H22Cl3N3O5
|
|||
| Canonical SMILES |
C1CC1C2=C(C(=NO2)C3=C(C=CC=C3Cl)Cl)COC4=CC(=C(C=C4)C5(CN(C5)C6=NC=CC(=C6)C(=O)O)O)Cl
|
|||
| InChI |
1S/C28H22Cl3N3O5/c29-20-2-1-3-21(30)24(20)25-18(26(39-33-25)15-4-5-15)12-38-17-6-7-19(22(31)11-17)28(37)13-34(14-28)23-10-16(27(35)36)8-9-32-23/h1-3,6-11,15,37H,4-5,12-14H2,(H,35,36)
|
|||
| InChIKey |
KZSKGLFYQAYZCO-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1418274-28-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Farnesoid X-activated receptor (FXR) | Target Info | Agonist | [3] |
| KEGG Pathway | Bile secretion | |||
| Pathway Interaction Database | RXR and RAR heterodimerization with other nuclear receptor | |||
| Reactome | Recycling of bile acids and salts | |||
| PPARA activates gene expression | ||||
| Endogenous sterols | ||||
| WikiPathways | Nuclear Receptors in Lipid Metabolism and Toxicity | |||
| Nuclear Receptors Meta-Pathway | ||||
| Farnesoid X Receptor Pathway | ||||
| Drug Induction of Bile Acid Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03890120) Safety, Tolerability, and Efficacy of Cilofexor in Non-Cirrhotic Adults With Primary Sclerosing Cholangitis (PRIMIS). U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT03449446) Study to Evaluate the Safety and Efficacy of Selonsertib, Firsocostat, Cilofexor, and Combinations in Participants With Bridging Fibrosis or Compensated Cirrhosis Due to Nonalcoholic Steatohepatitis (NASH) (ATLAS). U.S. National Institutes of Health. | |||
| REF 3 | The Nonsteroidal Farnesoid X Receptor Agonist Cilofexor (GS-9674) Improves Markers of Cholestasis and Liver Injury in Patients With Primary Sclerosing Cholangitis. Hepatology. 2019 Sep;70(3):788-801. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

