Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T51426
(Former ID: TTDS00272)
|
|||||
| Target Name |
Farnesoid X-activated receptor (FXR)
|
|||||
| Synonyms |
Retinoid X receptor-interacting protein 14; RXR-interacting protein 14; RIP14; Nuclear receptor subfamily 1 group H member 4; HRR1; Farnesol receptor HRR-1; FXR; Bile acid receptor; BAR
Click to Show/Hide
|
|||||
| Gene Name |
NR1H4
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Autoimmune liver disease [ICD-11: DB96] | |||||
| 2 | Cholelithiasis [ICD-11: DC11] | |||||
| 3 | Osteoarthritis [ICD-11: FA00-FA05] | |||||
| Function |
Ligand-activated transcription factor. Receptor for bile acids (BAs) such as chenodeoxycholic acid (CDCA), lithocholic acid, deoxycholic acid (DCA) and allocholic acid (ACA). Plays a essential role in BA homeostasis through the regulation of genes involved in BA synthesis, conjugation and enterohepatic circulation. Also regulates lipid and glucose homeostasis and is involved innate immune response. The FXR-RXR heterodimer binds predominantly to farnesoid X receptor response elements (FXREs) containing two inverted repeats of the consensus sequence 5'-AGGTCA-3' in which the monomers are spaced by 1 nucleotide (IR-1) but also to tandem repeat DR1 sites with lower affinity, and can be activated by either FXR or RXR-specific ligands. It is proposed that monomeric nuclear receptors such as NR5A2/LRH-1 bound to coregulatory nuclear responsive element (NRE) halfsites located in close proximity to FXREs modulate transcriptional activity (By similarity). In the liver activates transcription of the corepressor NR0B2 thereby indirectly inhibiting CYP7A1 and CYP8B1 (involved in BA synthesis) implicating at least in part histone demethylase KDM1A resulting in epigenomic repression, and SLC10A1/NTCP (involved in hepatic uptake of conjugated BAs). Activates transcription of the repressor MAFG (involved in regulation of BA synthesis) (By similarity). Activates transcription of SLC27A5/BACS and BAAT (involved in BA conjugation), ABCB11/BSEP (involved in bile salt export) by directly recruiting histone methyltransferase CARM1, and ABCC2/MRP2 (involved in secretion of conjugated BAs) and ABCB4 (involved in secretion of phosphatidylcholine in the small intestine). Activates transcription of SLC27A5/BACS and BAAT (involved in BA conjugation), ABCB11/BSEP (involved in bile salt export) by directly recruiting histone methyltransferase CARM1, and ABCC2/MRP2 (involved in secretion of conjugated BAs) and ABCB4 (involved in secretion of phosphatidylcholine in the small intestine). In the intestine activates FGF19 expression and secretion leading to hepatic CYP7A1 repression. The function also involves the coordinated induction of hepatic KLB/beta-klotho expression (By similarity). Regulates transcription of liver UGT2B4 and SULT2A1 involved in BA detoxification; binding to the UGT2B4 promoter seems to imply a monomeric transactivation independent of RXRA. Modulates lipid homeostasis by activating liver NR0B2/SHP-mediated repression of SREBF1 (involved in de novo lipogenesis), expression of PLTP (involved in HDL formation), SCARB1 (involved in HDL hepatic uptake), APOE, APOC1, APOC4, PPARA (involved in beta-oxidation of fatty acids), VLDLR and SDC1 (involved in the hepatic uptake of LDL and IDL remnants), and inhibiting expression of MTTP (involved in VLDL assembly. Increases expression of APOC2 (promoting lipoprotein lipase activity implicated in triglyceride clearance). Transrepresses APOA1 involving a monomeric competition with NR2A1 for binding to a DR1 element. Also reduces triglyceride clearance by inhibiting expression of ANGPTL3 and APOC3 (both involved in inhibition of lipoprotein lipase). Involved in glucose homeostasis by modulating hepatic gluconeogenesis through activation of NR0B2/SHP-mediated repression of respective genes. Modulates glycogen synthesis (inducing phosphorylation of glycogen synthase kinase-3) (By similarity). Modulates glucose-stimulated insulin secretion and is involved in insulin resistance. Involved in intestinal innate immunity. Plays a role in protecting the distal small intestine against bacterial overgrowth and preservation of the epithelial barrier (By similarity). Down-regulates inflammatory cytokine expression in several types of immune cells including macrophages and mononuclear cells. Mediates trans-repression of TLR4-induced cytokine expression; the function seems to require its sumoylation and prevents N-CoR nuclear receptor corepressor clearance from target genes such as IL1B and NOS2. Involved in the TLR9-mediated protective mechanism in intestinal inflammation. Plays an anti-inflammatory role in liver inflammation; proposed to inhibit proinflammatory (but not antiapoptotic) NF-kappa-B signaling) (By similarity).
Click to Show/Hide
|
|||||
| BioChemical Class |
Nuclear hormone receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MVMQFQGLENPIQISPHCSCTPSGFFMEMMSMKPAKGVLTEQVAGPLGQNLEVEPYSQYS
NVQFPQVQPQISSSSYYSNLGFYPQQPEEWYSPGIYELRRMPAETLYQGETEVAEMPVTK KPRMGASAGRIKGDELCVVCGDRASGYHYNALTCEGCKGFFRRSITKNAVYKCKNGGNCV MDMYMRRKCQECRLRKCKEMGMLAECMYTGLLTEIQCKSKRLRKNVKQHADQTVNEDSEG RDLRQVTSTTKSCREKTELTPDQQTLLHFIMDSYNKQRMPQEITNKILKEEFSAEENFLI LTEMATNHVQVLVEFTKKLPGFQTLDHEDQIALLKGSAVEAMFLRSAEIFNKKLPSGHSD LLEERIRNSGISDEYITPMFSFYKSIGELKMTQEEYALLTAIVILSPDRQYIKDREAVEK LQEPLLDVLQKLCKIHQPENPQHFACLLGRLTELRTFNHHHAEMLMSWRVNDHKFTPLLC EIWDVQ Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T24C0P | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 3 Approved Drugs | + | ||||
| 1 | Chenodiol | Drug Info | Approved | Cholelithiasis | [2] | |
| 2 | Guggulsterone | Drug Info | Approved | Osteoarthritis | [3], [4] | |
| 3 | obeticholic acid | Drug Info | Approved | Primary biliary cholangitis | [5] | |
| Clinical Trial Drug(s) | [+] 11 Clinical Trial Drugs | + | ||||
| 1 | Bevacizumab + Trastuzumab | Drug Info | Phase 3 | Breast cancer | [6] | |
| 2 | GS-9674 | Drug Info | Phase 3 | Primary sclerosing cholangitis | [7] | |
| 3 | Apomine | Drug Info | Phase 2 | Osteopetrosis | [8] | |
| 4 | EDP-305 | Drug Info | Phase 2 | Non-alcoholic steatohepatitis | [9] | |
| 5 | EYP001 | Drug Info | Phase 2 | Non-alcoholic steatohepatitis | [10] | |
| 6 | LJN452 | Drug Info | Phase 2 | Primary biliary cholangitis | [11] | |
| 7 | LMB763 | Drug Info | Phase 2 | Non-alcoholic steatohepatitis | [12] | |
| 8 | MET409 | Drug Info | Phase 2 | Non-alcoholic steatohepatitis | [13] | |
| 9 | AGN-242266 | Drug Info | Phase 1 | Non-alcoholic steatohepatitis | [14] | |
| 10 | PX-102 | Drug Info | Phase 1 | Hepatic fibrosis | [15] | |
| 11 | Turofexorate isopropyl | Drug Info | Phase 1 | Hyperlipidaemia | [16] | |
| Discontinued Drug(s) | [+] 2 Discontinued Drugs | + | ||||
| 1 | SB-756050 | Drug Info | Discontinued in Phase 2 | Type-2 diabetes | [17] | |
| 2 | SKF-97426 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [18] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | INT-767 | Drug Info | Preclinical | Hepatic fibrosis | [19] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Modulator | [+] 4 Modulator drugs | + | ||||
| 1 | Chenodiol | Drug Info | [20], [21] | |||
| 2 | obeticholic acid | Drug Info | [5] | |||
| 3 | Turofexorate isopropyl | Drug Info | [31] | |||
| 4 | SKF-97426 | Drug Info | [34] | |||
| Antagonist | [+] 3 Antagonist drugs | + | ||||
| 1 | Guggulsterone | Drug Info | [1], [22] | |||
| 2 | Bevacizumab + Trastuzumab | Drug Info | [20] | |||
| 3 | PMID29649907-Compound-44 | Drug Info | [32] | |||
| Agonist | [+] 60 Agonist drugs | + | ||||
| 1 | GS-9674 | Drug Info | [23] | |||
| 2 | Apomine | Drug Info | [24] | |||
| 3 | EDP-305 | Drug Info | [25] | |||
| 4 | EYP001 | Drug Info | [26] | |||
| 5 | LJN452 | Drug Info | [11], [27] | |||
| 6 | LMB763 | Drug Info | [28] | |||
| 7 | MET409 | Drug Info | [29] | |||
| 8 | AGN-242266 | Drug Info | [14] | |||
| 9 | PX-102 | Drug Info | [30] | |||
| 10 | PMID29649907-Compound-1 | Drug Info | [32] | |||
| 11 | PMID29649907-Compound-10 | Drug Info | [32] | |||
| 12 | PMID29649907-Compound-11 | Drug Info | [32] | |||
| 13 | PMID29649907-Compound-12 | Drug Info | [32] | |||
| 14 | PMID29649907-Compound-13 | Drug Info | [32] | |||
| 15 | PMID29649907-Compound-14 | Drug Info | [32] | |||
| 16 | PMID29649907-Compound-15 | Drug Info | [32] | |||
| 17 | PMID29649907-Compound-18 | Drug Info | [32] | |||
| 18 | PMID29649907-Compound-19 | Drug Info | [32] | |||
| 19 | PMID29649907-Compound-2 | Drug Info | [32] | |||
| 20 | PMID29649907-Compound-20 | Drug Info | [32] | |||
| 21 | PMID29649907-Compound-21 | Drug Info | [32] | |||
| 22 | PMID29649907-Compound-22 | Drug Info | [32] | |||
| 23 | PMID29649907-Compound-23 | Drug Info | [32] | |||
| 24 | PMID29649907-Compound-24 | Drug Info | [32] | |||
| 25 | PMID29649907-Compound-25 | Drug Info | [32] | |||
| 26 | PMID29649907-Compound-26 | Drug Info | [32] | |||
| 27 | PMID29649907-Compound-27 | Drug Info | [32] | |||
| 28 | PMID29649907-Compound-28 | Drug Info | [32] | |||
| 29 | PMID29649907-Compound-29 | Drug Info | [32] | |||
| 30 | PMID29649907-Compound-3 | Drug Info | [32] | |||
| 31 | PMID29649907-Compound-30 | Drug Info | [32] | |||
| 32 | PMID29649907-Compound-31 | Drug Info | [32] | |||
| 33 | PMID29649907-Compound-32 | Drug Info | [32] | |||
| 34 | PMID29649907-Compound-33 | Drug Info | [32] | |||
| 35 | PMID29649907-Compound-34 | Drug Info | [32] | |||
| 36 | PMID29649907-Compound-35 | Drug Info | [32] | |||
| 37 | PMID29649907-Compound-36 | Drug Info | [32] | |||
| 38 | PMID29649907-Compound-37 | Drug Info | [32] | |||
| 39 | PMID29649907-Compound-38 | Drug Info | [32] | |||
| 40 | PMID29649907-Compound-39 | Drug Info | [32] | |||
| 41 | PMID29649907-Compound-4 | Drug Info | [32] | |||
| 42 | PMID29649907-Compound-40 | Drug Info | [32] | |||
| 43 | PMID29649907-Compound-41 | Drug Info | [32] | |||
| 44 | PMID29649907-Compound-42 | Drug Info | [32] | |||
| 45 | PMID29649907-Compound-5 | Drug Info | [32] | |||
| 46 | PMID29649907-Compound-8 | Drug Info | [32] | |||
| 47 | PMID29649907-Compound-9 | Drug Info | [32] | |||
| 48 | PMID29649907-Compound-INT767 | Drug Info | [32] | |||
| 49 | PMID30259754-Compound-INT-767 | Drug Info | [27] | |||
| 50 | PMID30259754-Compound-LY2562175 | Drug Info | [27] | |||
| 51 | PMID30259754-Compound-Px-102 | Drug Info | [27] | |||
| 52 | PMID30259754-Compound-pyrrole[2,3-d]azepines | Drug Info | [27] | |||
| 53 | PMID30259754-Compound-WAY-362450 | Drug Info | [27] | |||
| 54 | SB-756050 | Drug Info | [33] | |||
| 55 | INT-767 | Drug Info | [35] | |||
| 56 | 1,1-bisphosphonate esters | Drug Info | [24] | |||
| 57 | 22R-hydroxycholesterol | Drug Info | [36] | |||
| 58 | cholesten | Drug Info | [38] | |||
| 59 | desmosterol | Drug Info | [38] | |||
| 60 | GW4065 | Drug Info | [20] | |||
| Inhibitor | [+] 3 Inhibitor drugs | + | ||||
| 1 | 12-O-deacetyl-12-epi-19-deoxy-21-hydroxyscalarin | Drug Info | [22] | |||
| 2 | AGN-34 | Drug Info | [37] | |||
| 3 | Fexaramine | Drug Info | [39] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Chenodiol | Ligand Info | |||||
| Structure Description | Crystal Structure of Farnesoid X receptor (FXR) with bound NCoA-2 peptide and CDCA | PDB:6HL1 | ||||
| Method | X-ray diffraction | Resolution | 1.60 Å | Mutation | No | [40] |
| PDB Sequence |
HMELTPDQQT
251 LLHFIMDSYN261 KQRMPQEITN271 KILKEEFSAE281 ENFLILTEMA291 TNHVQVLVEF 301 TKKLPGFQTL311 DHEDQIALLK321 GSAVEAMFLR331 SAEIFNKSDL347 LEERIRNSGI 357 SDEYITPMFS367 FYKSIGELKM377 TQEEYALLTA387 IVILSPDRQY397 IKDREAVEKL 407 QEPLLDVLQK417 LCKIHQPENP427 QHFACLLGRL437 TELRTFNHHH447 AEMLMSWRVN 457 DHKFTPLLCE467 IWDVQ
|
|||||
|
|
MET265
3.606
LEU287
3.962
MET290
3.273
ALA291
3.706
HIS294
3.571
MET328
3.617
PHE329
3.696
ARG331
3.193
SER332
2.878
ILE335
3.492
PHE336
4.160
|
|||||
| Ligand Name: Ivermectin | Ligand Info | |||||
| Structure Description | Identification of a novel FXR ligand that regulates metabolism | PDB:4WVD | ||||
| Method | X-ray diffraction | Resolution | 2.90 Å | Mutation | No | [41] |
| PDB Sequence |
ELTPDQQTLL
253 HFIMDSYNKQ263 RMPQEITNKI273 LKEFSAEENF284 LILTEMATNH294 VQVLVEFTKK 304 LPGFQTLDHE314 DQIALLKGSA324 VEAMFLRSAE334 IFNKKLPSGH344 SDLLEERIRN 354 SGISDEYITP364 MFSFYKSIGE374 LKMTQEEYAL384 LTAIVILSPD394 RQYIKDREAV 404 EKLQEPLLDV414 LQKLCKIHQP424 ENPQHFACLL434 GRLTELRTFN444 HHHAEMLMSW 454
|
|||||
|
|
GLN263
4.634
ARG264
3.050
LYS272
3.674
ILE273
4.481
GLU276
3.437
SER279
4.395
ASN283
2.985
LEU287
3.633
MET290
2.720
ALA291
3.624
ASN293
2.619
HIS294
3.589
VAL297
4.281
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
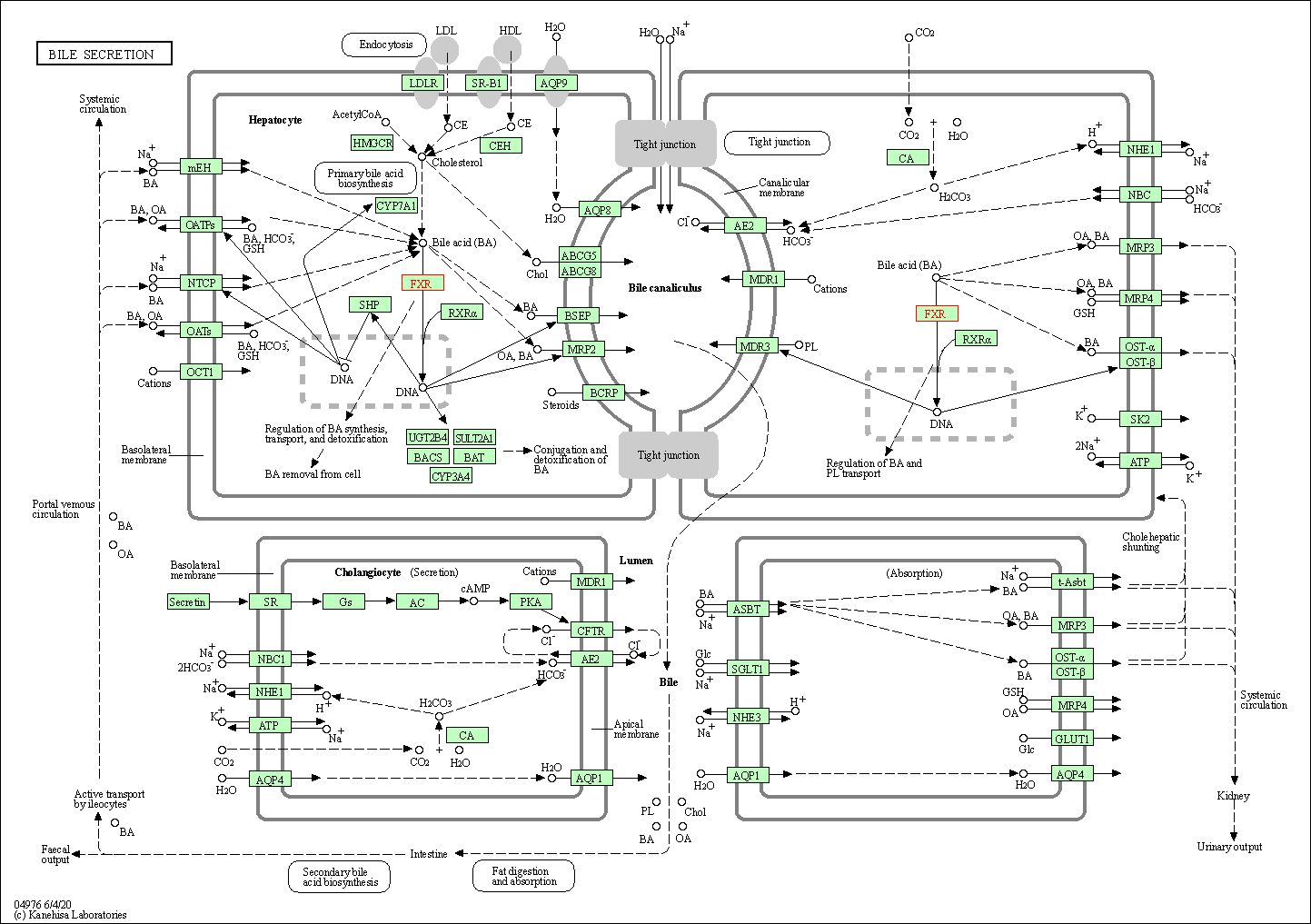
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Bile secretion | hsa04976 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Degree | 6 | Degree centrality | 6.45E-04 | Betweenness centrality | 4.65E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.98E-01 | Radiality | 1.34E+01 | Clustering coefficient | 1.33E-01 |
| Neighborhood connectivity | 1.57E+01 | Topological coefficient | 2.61E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 1 KEGG Pathways | + | ||||
| 1 | Bile secretion | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | RXR and RAR heterodimerization with other nuclear receptor | |||||
| Reactome | [+] 3 Reactome Pathways | + | ||||
| 1 | Recycling of bile acids and salts | |||||
| 2 | PPARA activates gene expression | |||||
| 3 | Endogenous sterols | |||||
| WikiPathways | [+] 4 WikiPathways | + | ||||
| 1 | Nuclear Receptors in Lipid Metabolism and Toxicity | |||||
| 2 | Nuclear Receptors Meta-Pathway | |||||
| 3 | Farnesoid X Receptor Pathway | |||||
| 4 | Drug Induction of Bile Acid Pathway | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Effect of guggulsterone and cembranoids of Commiphora mukul on pancreatic phospholipase A(2): role in hypocholesterolemia. J Nat Prod. 2009 Jan;72(1):24-8. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2745). | |||||
| REF 4 | Treating insomnia: Current and investigational pharmacological approaches. Pharmacol Ther. 2006 Dec;112(3):612-29. | |||||
| REF 5 | 2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73-76. | |||||
| REF 6 | AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013 May 10;31(14):1719-25. | |||||
| REF 7 | ClinicalTrials.gov (NCT03890120) Safety, Tolerability, and Efficacy of Cilofexor in Non-Cirrhotic Adults With Primary Sclerosing Cholangitis (PRIMIS). U.S. National Institutes of Health. | |||||
| REF 8 | A phase II open-label trial of apomine (SR-45023A) in patients with refractory melanoma. Invest New Drugs. 2006 Jan;24(1):89-94. | |||||
| REF 9 | ClinicalTrials.gov (NCT04378010) A Randomized, Double-blind Study to Assess the Safety and Efficacy of EDP-305 in Subjects With Liver-biopsy Proven NASH. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT03812029) Safety, Tolerability, Pharmacokinetics and Efficacy of EYP001a in Patients With Nonalcoholic Steatohepatitis (NASH). U.S. National Institutes of Health. | |||||
| REF 11 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 12 | ClinicalTrials.gov (NCT02913105) Safety, Tolerability, Pharmacokinetics and Efficacy of LMB763 in Patients With NASH. U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT04702490) Study to Evaluate MET409 Alone or in Combination With Empagliflozin in Patients With Type 2 Diabetes and NASH. U.S. National Institutes of Health. | |||||
| REF 14 | Clinical pipeline report, company report or official report of AbbVie. | |||||
| REF 15 | ClinicalTrials.gov (NCT01998659) Single Ascending Oral Dose Phase I Study With Px-102. U.S. National Institutes of Health. | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025892) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028284) | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002680) | |||||
| REF 19 | Clinical pipeline report, company report or official report of Intercept. | |||||
| REF 20 | Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem. 2002 Aug 30;277(35):31441-7. | |||||
| REF 21 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 22 | Scalarane sesterterpenes from a marine sponge of the genus Spongia and their FXR antagonistic activity. J Nat Prod. 2007 Nov;70(11):1691-5. | |||||
| REF 23 | The Nonsteroidal Farnesoid X Receptor Agonist Cilofexor (GS-9674) Improves Markers of Cholestasis and Liver Injury in Patients With Primary Sclerosing Cholangitis. Hepatology. 2019 Sep;70(3):788-801. | |||||
| REF 24 | The nuclear receptors FXR and LXRalpha: potential targets for the development of drugs affecting lipid metabolism and neoplastic diseases. Curr Pharm Des. 2001 Mar;7(4):231-59. | |||||
| REF 25 | A novel non-bile acid FXR agonist EDP-305 potently suppresses liver injury and fibrosis without worsening of ductular reaction. Liver Int. 2020 Jul;40(7):1655-1669. | |||||
| REF 26 | Clinical pipeline report, company report or official report of ENYO Pharma. | |||||
| REF 27 | FXR modulators for enterohepatic and metabolic diseases.Expert Opin Ther Pat. 2018 Nov;28(11):765-782. | |||||
| REF 28 | Nidufexor (LMB763), a Novel FXR Modulator for the Treatment of Nonalcoholic Steatohepatitis. J Med Chem. 2020 Apr 23;63(8):3868-3880. | |||||
| REF 29 | Clinical pipeline report, company report or official report of Metacrine. | |||||
| REF 30 | An FXR Agonist Reduces Bile Acid Synthesis Independently of Increases in FGF19 in Healthy Volunteers. Gastroenterology. 2018 Oct;155(4):1012-1016. | |||||
| REF 31 | A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am J Physiol Gastrointest Liver Physiol. 2009 Mar;296(3):G543-52. | |||||
| REF 32 | Farnesoid X receptor modulators 2014-present: a patent review.Expert Opin Ther Pat. 2018 May;28(5):351-364. | |||||
| REF 33 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 34 | SK&F 97426-A a more potent bile acid sequestrant and hypocholesterolaemic agent than cholestyramine in the hamster. Atherosclerosis. 1993 Jun;101(1):51-60. | |||||
| REF 35 | FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J Am Soc Nephrol. 2018 Jan;29(1):118-137. | |||||
| REF 36 | Oxysterol 22(R)-hydroxycholesterol induces the expression of the bile salt export pump through nuclear receptor farsenoid X receptor but not liver ... J Pharmacol Exp Ther. 2006 Apr;317(1):317-25. | |||||
| REF 37 | Farnesoid X receptor: from structure to potential clinical applications. J Med Chem. 2005 Aug 25;48(17):5383-403. | |||||
| REF 38 | Identification of farnesoid X receptor beta as a novel mammalian nuclear receptor sensing lanosterol. Mol Cell Biol. 2003 Feb;23(3):864-72. | |||||
| REF 39 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 40 | Molecular tuning of farnesoid X receptor partial agonism. Nat Commun. 2019 Jul 2;10(1):2915. | |||||
| REF 41 | The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism. Nat Commun. 2013;4:1937. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

