Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D7LQJ8
|
|||
| Drug Name |
PX-102
|
|||
| Synonyms |
Px-102; PX20606 trans-isomer; UNII-378SU5NO8S; 378SU5NO8S; CHEMBL3822773; 1268244-85-4 (trans-isomer); 4-((1S,2S)-2-(2-CHLORO-4-((5-CYCLOPROPYL-3-(2,6-DICHLOROPHENYL)ISOXAZOL-4-YL)METHOXY)PHENYL)CYCLOPROPYL)BENZOIC ACID; Px-104; 2020096-17-5; 1268244-85-4; PX 20606; SCHEMBL17087854; 4-(2-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-1,2-oxazol-4-yl)methoxy)phenyl)cyclopropyl)benzoic acid; BDBM50185707; ZINC115372389; DB15416; 4-(2-(2-Chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-4-isoxazolyl)methoxy)phenyl)cyclopropyl)benzoic acid; AC-30349; PX-20606; PX-102(PX-20606); UNII-6TU6SUZ3BY component XBUXXJUEBFDQHD-NHCUHLMSSA-N; Benzoic acid, 4-((1R,2R)-2-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-4-isoxazolyl)methoxy)phenyl)cyclopropyl)-, rel-(-)-; Benzoic acid, 4-(2-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-4-isoxazolyl)methoxy)phenyl)cyclopropyl)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hepatic fibrosis [ICD-11: DB93.0; ICD-10: K74.0; ICD-9: 709.2] | Phase 1 | [1] | |
| Structure |
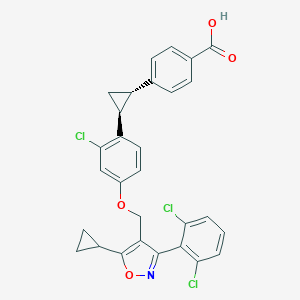 |
Download2D MOL |
||
| Formula |
C29H22Cl3NO4
|
|||
| Canonical SMILES |
C1CC1C2=C(C(=NO2)C3=C(C=CC=C3Cl)Cl)COC4=CC(=C(C=C4)C5CC5C6=CC=C(C=C6)C(=O)O)Cl
|
|||
| InChI |
1S/C29H22Cl3NO4/c30-23-2-1-3-24(31)26(23)27-22(28(37-33-27)16-6-7-16)14-36-18-10-11-19(25(32)12-18)21-13-20(21)15-4-8-17(9-5-15)29(34)35/h1-5,8-12,16,20-21H,6-7,13-14H2,(H,34,35)/t20-,21-/m1/s1
|
|||
| InChIKey |
XBUXXJUEBFDQHD-NHCUHLMSSA-N
|
|||
| CAS Number |
CAS 1268244-85-4
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Farnesoid X-activated receptor (FXR) | Target Info | Agonist | [2] |
| KEGG Pathway | Bile secretion | |||
| Pathway Interaction Database | RXR and RAR heterodimerization with other nuclear receptor | |||
| Reactome | Recycling of bile acids and salts | |||
| PPARA activates gene expression | ||||
| Endogenous sterols | ||||
| WikiPathways | Nuclear Receptors in Lipid Metabolism and Toxicity | |||
| Nuclear Receptors Meta-Pathway | ||||
| Farnesoid X Receptor Pathway | ||||
| Drug Induction of Bile Acid Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01998659) Single Ascending Oral Dose Phase I Study With Px-102. U.S. National Institutes of Health. | |||
| REF 2 | An FXR Agonist Reduces Bile Acid Synthesis Independently of Increases in FGF19 in Healthy Volunteers. Gastroenterology. 2018 Oct;155(4):1012-1016. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

