Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00CVJ
|
|||
| Former ID |
DNCL003143
|
|||
| Drug Name |
Neu-P11
|
|||
| Synonyms |
Piromelatine; UNII-S3UN2146K9; 946846-83-9; S3UN2146K9; NEU-P11; Piromelatine [INN]; NEU-P-11; SCHEMBL8235551; DTXSID90241566; N-(2-(5-Methoxy-1H-indol-3-yl)ethyl)-4-oxo-4H-pyran-2-carboxamide; 4H-Pyran-2-carboxamide, N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-4-oxo-; SB19819
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 2 | [1], [2] | |
| Dementia [ICD-11: 6D80-6D86] | Phase 2 | [2] | ||
| Insomnia [ICD-11: 7A00-7A0Z] | Phase 2 | [3] | ||
| Company |
Neurim Pharmaceuticals
|
|||
| Structure |
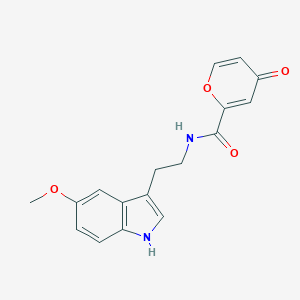 |
Download2D MOL |
||
| Formula |
C17H16N2O4
|
|||
| Canonical SMILES |
COC1=CC2=C(C=C1)NC=C2CCNC(=O)C3=CC(=O)C=CO3
|
|||
| InChI |
1S/C17H16N2O4/c1-22-13-2-3-15-14(9-13)11(10-19-15)4-6-18-17(21)16-8-12(20)5-7-23-16/h2-3,5,7-10,19H,4,6H2,1H3,(H,18,21)
|
|||
| InChIKey |
PNTNBIHOAPJYDB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 946846-83-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 1A receptor (HTR1A) | Target Info | Agonist | [4] |
| 5-HT 1D receptor (HTR1D) | Target Info | Agonist | [4] | |
| Melatonin receptor type 1A (MTNR1A) | Target Info | Agonist | [5] | |
| KEGG Pathway | cAMP signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Serotonergic synapse | ||||
| Circadian entrainment | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| 5HT1 type receptor mediated signaling pathway | ||||
| Reactome | Serotonin receptors | |||
| G alpha (i) signalling events | ||||
| Class A/1 (Rhodopsin-like receptors) | ||||
| WikiPathways | Serotonin HTR1 Group and FOS Pathway | |||
| SIDS Susceptibility Pathways | ||||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| Small Ligand GPCRs | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | ClinicalTrials.gov (NCT01489969) Sleep Laboratory Study to Investigate the Safety and Efficacy of Neu-P11 in Primary Insomnia Patients. U.S. National Institutes of Health. | |||
| REF 4 | Clinical pipeline report, company report or official report of Neurim Pharmaceuticals. | |||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 288). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

