Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00HBR
|
|||
| Former ID |
DIB015545
|
|||
| Drug Name |
AIKO-150
|
|||
| Synonyms |
6beta-Naltrexol; beta-Naltrexol; 6beta-Hydroxynaltrexone; 6alpha-Hydroxynaltrexone; 49625-89-0; 6alpha-Naltrexol; UNII-J0W963M37T; CHEMBL140278; J0W963M37T; naltrexol; Naltrexone-6-beta-ol; alpha-Naltrexol; 6beta-Naltrexone; 6alpha-Naltrexone; 6-alpha-naltrexol; N-Cyclopropylmethyl-7,8-dihydro-14-hydroxynorisomorphine; SCHEMBL679700; DTXSID80197942; JLVNEHKORQFVQJ-PYIJOLGTSA-N; ZINC6092289; BDBM50001709; NCGC00165851-01; FT-0672600; Opioid neutral antagonists (iv, pain), Aiko Biotechnology; 6-beta-naltrexol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Opioid dependence [ICD-11: 6C43.2Z; ICD-9: 304] | Phase 1 | [1] | |
| Company |
Aiko Biotechnology Inc
|
|||
| Structure |
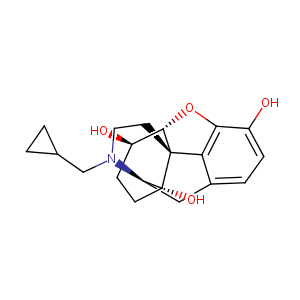 |
Download2D MOL |
||
| Formula |
C20H25NO4
|
|||
| Canonical SMILES |
C1CC1CN2CCC34C5C(CCC3(C2CC6=C4C(=C(C=C6)O)O5)O)O
|
|||
| InChI |
1S/C20H25NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,14-15,18,22-24H,1-2,5-10H2/t14-,15-,18+,19+,20-/m1/s1
|
|||
| InChIKey |
JLVNEHKORQFVQJ-PYIJOLGTSA-N
|
|||
| CAS Number |
CAS 49625-89-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Opioid receptor delta (OPRD1) | Target Info | Inhibitor | [2] |
| Opioid receptor kappa (OPRK1) | Target Info | Inhibitor | [2] | |
| Opioid receptor mu (MOP) | Target Info | Antagonist | [2], [3] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Estrogen signaling pathway | ||||
| Morphine addiction | ||||
| cGMP-PKG signaling pathway | ||||
| Sphingolipid signaling pathway | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Enkephalin release | ||||
| Opioid proenkephalin pathway | ||||
| Opioid proopiomelanocortin pathway | ||||
| Opioid prodynorphin pathway | ||||
| Pathway Interaction Database | IL4-mediated signaling events | |||
| Reactome | Peptide ligand-binding receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | TCR Signaling Pathway | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Peptide GPCRs | ||||
| Opioid Signalling | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00829777) Safety Study of Intravenous 6 Naltrexol (AIKO-150) in Opioid-Dependent Subjects. U.S. National Institutes of Health. | |||
| REF 2 | Syntheses and opioid receptor binding properties of carboxamido-substituted opioids. Bioorg Med Chem Lett. 2009 Jan 1;19(1):203-8. | |||
| REF 3 | Clinical pipeline report, company report or official report of signaturerx. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

