Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05TIB
|
|||
| Former ID |
DAP000342
|
|||
| Drug Name |
Trospium
|
|||
| Synonyms |
Spasmex; Spasmoplex; Trosec; Regurin (TN); Sanctura (TN); Spasmex (TN); Spasmoplex (TN); Trosec (TN); Trospium chloride (JAN/USAN/INN); Spiro(8-azoniabicyclo(3.2.1)octane-8,1'-pyrrolidinium), 3-hydroxy-, chloride, benzilate; Spiro[8-azoniabicyclo[3.2.1]octane-8,1'-azolidin-1-ium]-3-yl 2-hydroxy-2,2-diphenylacetate; Spiro[8-azoniabicyclo[3.2.1]octane-8,1'-azolidin-1-ium]-3-yl 2-hydroxy-2,2-diphenylacetate chloride; 3-Hydroxyspiro(8-azoniabicyclo(3.2.1)octane-8,1'-pyrrolidinium) chloride benzilate; 6,10-Ethano-5-azoniaspiro(4.5)decan-8-ol, chloride, benzilate; 8-Benziloyloxy-6,10-ethano-5-azoniaspiro(4.5)decane chloride
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Overactive bladder [ICD-11: GC50.0; ICD-10: N32.8] | Approved | [1], [2], [3], [4], [5] | |
| Spasm [ICD-11: MB47.3; ICD-10: R25.2] | Approved | [1], [2], [3], [4], [5] | ||
| Therapeutic Class |
Parasympatholytics
|
|||
| Company |
Allergan Pharmaceuticals
|
|||
| Structure |
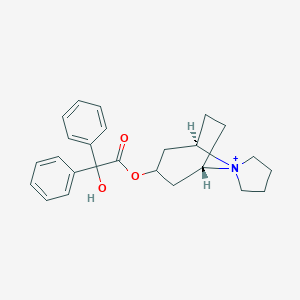 |
Download2D MOL |
||
| Formula |
C25H30NO3+
|
|||
| Canonical SMILES |
C1CC[N+]2(C1)C3CCC2CC(C3)OC(=O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O
|
|||
| InChI |
1S/C25H30NO3/c27-24(25(28,19-9-3-1-4-10-19)20-11-5-2-6-12-20)29-23-17-21-13-14-22(18-23)26(21)15-7-8-16-26/h1-6,9-12,21-23,28H,7-8,13-18H2/q+1/t21-,22+,23?
|
|||
| InChIKey |
OYYDSUSKLWTMMQ-AIZNXBIQSA-N
|
|||
| CAS Number |
CAS 47608-32-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:145791
|
|||
| ADReCS Drug ID | BADD_D02305 | |||
| SuperDrug ATC ID |
G04BD09
|
|||
| SuperDrug CAS ID |
cas=010405024
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7480). | |||
| REF 2 | 2004 approvals: the demise of the blockbuster. Nat Rev Drug Discov. 2005 Feb;4(2):93-4. | |||
| REF 3 | Trospium chloride: the European experience. Expert Opin Pharmacother. 2006 Jul;7(10):1373-80. | |||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 5 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021595. | |||
| REF 6 | Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

