Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06BYS
|
|||
| Former ID |
DIB003127
|
|||
| Drug Name |
Imepitoin
|
|||
| Synonyms |
ADD-233089; AWD-131-138
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Convulsion [ICD-11: 8A68.Z; ICD-9: 780.3] | Discontinued in Phase 1 | [1] | |
| Company |
ASTA Medica AG
|
|||
| Structure |
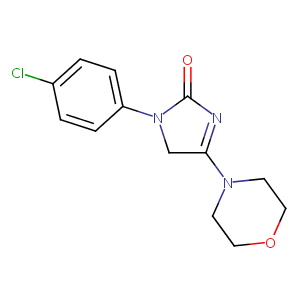 |
Download2D MOL |
||
| Formula |
C13H14ClN3O2
|
|||
| Canonical SMILES |
C1COCCN1C2=NC(=O)N(C2)C3=CC=C(C=C3)Cl
|
|||
| InChI |
1S/C13H14ClN3O2/c14-10-1-3-11(4-2-10)17-9-12(15-13(17)18)16-5-7-19-8-6-16/h1-4H,5-9H2
|
|||
| InChIKey |
IQHYCZKIFIHTAI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 188116-07-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Translocator protein (TSPO) | Target Info | Agonist | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| HTLV-I infection | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008368) | |||
| REF 2 | The pharmacology of imepitoin: the first partial benzodiazepine receptor agonist developed for the treatment of epilepsy. CNS Drugs. 2014 Jan;28(1):29-43. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

