Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06RCB
|
|||
| Former ID |
DAP000914
|
|||
| Drug Name |
Diethylcarbamazine
|
|||
| Synonyms |
Bitirazine; Camin; Caracide; Carbamazine; Carbilazine; Caricide; Cypip; Decacide; Diaethylcarbamazinum; Diethylcarbamazinum; Dietilcarbamazina; Ethodryl; Luxuran; Notezine; Spatonin; Diethyl carbamazine; Ditrazine Base; Banocide (TN); Camin (TN); Carbilazine (TN); Caricide (TN); Cypip (TN); Diethylcarbamazine (INN); Diethylcarbamazine [INN:BAN]; Diethylcarbamazinum [INN-Latin]; Dietilcarbamazina [INN-Spanish]; Ethodryl (TN); FR-1031; Filaribits (TN); Forte (TN); Hetrazan (TN); Spatonin (TN); N,N-Diethyl-4-methyl-1-piperazinecarboxamide; N,N-diethyl-4-methylpiperazine-1-carboxamide; 1-Diethylcarbamyl-4-methylpiperazine; 84L
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Lymphatic filariasis [ICD-11: 1F66.3; ICD-9: 125] | Approved | [1], [2] | |
| Therapeutic Class |
Anthelmintics
|
|||
| Company |
Lederle Laboratories Div American Cyanamid Co
|
|||
| Structure |
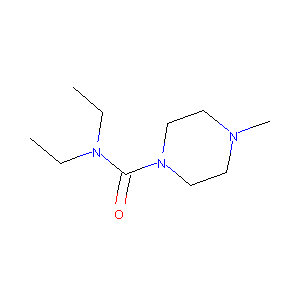 |
Download2D MOL |
||
| Formula |
C10H21N3O
|
|||
| Canonical SMILES |
CCN(CC)C(=O)N1CCN(CC1)C
|
|||
| InChI |
1S/C10H21N3O/c1-4-12(5-2)10(14)13-8-6-11(3)7-9-13/h4-9H2,1-3H3
|
|||
| InChIKey |
RCKMWOKWVGPNJF-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 90-89-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10169, 603056, 5635251, 7979075, 8151942, 10537738, 11335723, 11360962, 11363832, 11366394, 11368956, 11371499, 11374220, 11377118, 11461934, 11466312, 11467432, 11484939, 11486143, 11488838, 11490206, 11492326, 11494752, 15414101, 29199083, 29222198, 46506830, 47216750, 47216751, 47216752, 47440221, 47515292, 47810725, 47885379, 48415888, 49698856, 50011676, 50123452, 50123453, 50263820, 57321580, 85788399, 96024522, 103191069, 103905855, 104302387, 107815220, 118238838, 124596531, 125623992
|
|||
| ChEBI ID |
CHEBI:4527
|
|||
| SuperDrug ATC ID |
P02CB02
|
|||
| SuperDrug CAS ID |
cas=000090891
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 5-lipoxygenase (5-LOX) | Target Info | Inhibitor | [3] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Vitamin D Receptor Pathway | |||
| Arachidonic acid metabolism | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Opportunities and challenges in antiparasitic drug discovery. Nat Rev Drug Discov. 2005 Sep;4(9):727-40. | |||
| REF 3 | Inhibition of leukotriene formation by diethylcarbamazine modifies the acid-base balance in the rabbits with blast injuries of the lungs. Vojnosanit Pregl. 1999 May-Jun;56(3):243-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

