Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06XRQ
|
|||
| Former ID |
DNC000128
|
|||
| Drug Name |
AA-861
|
|||
| Synonyms |
aa-861; DOCEBENONE; 80809-81-0; Docebenonum; Docebenona; AA861; Docebenone [USAN:INN]; Docebenonum [INN-Latin]; Docebenona [INN-Spanish]; UNII-2XRX3BD53M; 2-(12-hydroxydodeca-5,10-diyn-1-yl)-3,5,6-trimethylcyclohexa-2,5-diene-1,4-dione; CHEBI:2340; A-61589; 2-(12-Hydroxy-5,10-dodecadiynyl)-3,5,6-trimethyl-p-benzoquinone; 2,3,5-trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone; 2XRX3BD53M; MLS000028467; A 61589; NCGC00015053-05; SMR000058412; 2-(12-hydroxydodeca-5,10-diynyl)-3,5,6-trimethyl-1,4-benzoquinone
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Allergy [ICD-11: 4A80-4A85; ICD-10: T78.4; ICD-9: 995.3] | Terminated | [1] | |
| Structure |
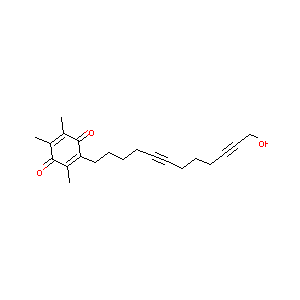 |
Download2D MOL |
||
| Formula |
C21H26O3
|
|||
| Canonical SMILES |
CC1=C(C(=O)C(=C(C1=O)C)CCCCC#CCCCC#CCO)C
|
|||
| InChI |
1S/C21H26O3/c1-16-17(2)21(24)19(18(3)20(16)23)14-12-10-8-6-4-5-7-9-11-13-15-22/h22H,5,7-10,12,14-15H2,1-3H3
|
|||
| InChIKey |
WDEABJKSGGRCQA-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 80809-81-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
4554, 856019, 5187752, 8148222, 8151355, 11110733, 11121818, 11122298, 11363020, 11365582, 11368144, 11371171, 11371172, 11373745, 11376306, 12013076, 14875022, 17397966, 17404590, 24278214, 26751594, 29221156, 47736598, 47885516, 48334611, 50065238, 50100163, 50104244, 50104245, 50104246, 50169859, 53777132, 53790839, 57321075, 85083177, 85230904, 90341036, 91702928, 92126158, 92303592, 92308997, 92310169, 99301081, 99302601, 103102927, 103268050, 104299228, 117584140, 121360897, 124749392
|
|||
| ChEBI ID |
CHEBI:2340
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 5-lipoxygenase (5-LOX) | Target Info | Inhibitor | [2], [3], [4], [5] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Vitamin D Receptor Pathway | |||
| Arachidonic acid metabolism | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000099) | |||
| REF 2 | Platelets stimulate airway smooth muscle cell proliferation through mechanisms involving 5-lipoxygenase and reactive oxygen species. Platelets. 2008 Nov;19(7):528-36. | |||
| REF 3 | Leukotriene B4/leukotriene B4 receptor pathway is involved in hepatic microcirculatory dysfunction elicited by endotoxin. Shock. 2008 Jul;30(1):87-91. | |||
| REF 4 | Effect of 5-lipoxygenase inhibitor on experimental delayed cerebral vasospasm. Stroke. 1987 Mar-Apr;18(2):512-8. | |||
| REF 5 | Characterization and modulation of antigen-induced effects in isolated rat heart. J Cardiovasc Pharmacol. 1991 Oct;18(4):556-65. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

