Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07MVK
|
|||
| Former ID |
DAP000953
|
|||
| Drug Name |
Halofantrine
|
|||
| Synonyms |
Halfan; Halofantrino; HALOFANTRINE HYDROCHLORIDE; Halofantrine HCl; Halofantrine Hydrochloride [USAN]; Halofantrine [USAN]; Halofantrino [Spanish]; WR 171669; Dl-WR 171669; Halfan (TN); Halofantrine hydrochloride (USAN); WR 171,699; WR-171669; WR-171699; SK&F-102866; WR-171,669; (+-)-Halofantrine hydrochloride; (1)-Halofantrine; 1,3-Dichloro-.alpha.-[2-(dibutylamino)ethyl]-6-(trifluoromethyl)-9-phenanthrene-methanol; 1,3-Dichloro-.alpha.-[2-(dibutylamino)ethyl]-6-(trifluoromethyl)-9-phenathrenemethanol; 1,3-Dichloro-6-trifluoromethyl-9-(3-(dibutylamino)-1-hydroxypropyl)phenanthrene HCl; 1,3-Dichloro-alpha-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-9-phenanthrenemethanol hydrochloride; 1,3-Dichloro-alpha-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)phenanthren-1-methanol hydrochloride; 1-(1,3-Dichloro-6-trifluoromethyl-9-phenanthryl)-3-(di-n-butylamino)propanol hydrochloride; 1-(1,3-dichloro-6-trifluoromethyl-9-phenanthryl)-3-di(n-butyl)aminopropanol HCl; 3-(Dibutylamino)-1-(1,3-dichloro-6-(trifluoromethyl)phenanthren-9-yl)propan-1-ol hydrochloride; 3-(dibutylamino)-1-[1,3-dichloro-6-(trifluoromethyl)phenanthren-9-yl]propan-1-ol; 3-(dibutylamino)-1-[1,3-dichloro-6-(trifluoromethyl)phenanthren-9-yl]propan-1-ol hydrochloride; 9-(3-(dibutylamino)-1-hydroxypropyl)-1,3-dichloro-6-(trifluoromethyl)phenanthrene hydrochloride; 9-Phenanthrenemethanol, 1,3-dichloro-.alpha.-[2-(dibutylamino)ethyl]-6-(trifluoromethyl)-(1); 9-Phenanthrenemethanol, 1,3-dichloro-alpha-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-, hydrochloride; 9-Phenanthrenemethanol, 1,3-dichloro-alpha-(2-(dibutylamino)ethyl)-6-(trifluoromethyl)-,hydrochloride
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Malaria [ICD-11: 1F40-1F45; ICD-10: B50-B64, B54] | Approved | [1] | |
| Therapeutic Class |
Antimalarials
|
|||
| Company |
GlaxoSmithKline
|
|||
| Structure |
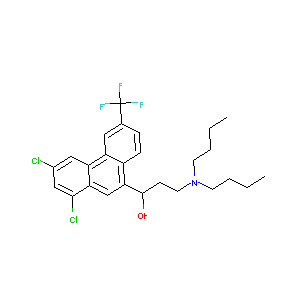 |
Download2D MOL |
||
| Formula |
C26H30Cl2F3NO
|
|||
| Canonical SMILES |
CCCCN(CCCC)CCC(C1=C2C=CC(=CC2=C3C=C(C=C(C3=C1)Cl)Cl)C(F)(F)F)O
|
|||
| InChI |
1S/C26H30Cl2F3NO/c1-3-5-10-32(11-6-4-2)12-9-25(33)23-16-22-21(14-18(27)15-24(22)28)20-13-17(26(29,30)31)7-8-19(20)23/h7-8,13-16,25,33H,3-6,9-12H2,1-2H3
|
|||
| InChIKey |
FOHHNHSLJDZUGQ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 69756-53-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9836, 603092, 4441467, 7979519, 8174993, 11406986, 11467059, 11468179, 11486828, 14859927, 34678697, 46506753, 47572921, 47572922, 47869196, 48317942, 48416078, 49699380, 50070734, 50123958, 77799953, 87245098, 92719358, 96024726, 103305814, 103994306, 104326444, 117505175, 125356362, 126424569, 126620878, 126653035, 126670922, 127875900, 134338227, 135029301, 135329562, 137251553, 152047097, 160643701, 160964551, 162178795, 163414203, 164786759, 172883008, 174006943, 179117060, 184545611, 196110120, 223400586
|
|||
| ChEBI ID |
CHEBI:94392
|
|||
| ADReCS Drug ID | BADD_D01053 | |||
| SuperDrug ATC ID |
P01BX01
|
|||
| SuperDrug CAS ID |
cas=069756532
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Eubacterium eligens
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Eubacterium eligens was decreased by Halofantrine hydrochloride (adjusted p-values: 1.23E-03). | |||
|
Studied Microbe: Eubacterium rectale
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Eubacterium rectale was decreased by Halofantrine hydrochloride (adjusted p-values: 2.28E-03). | |||
|
Studied Microbe: Roseburia hominis
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Roseburia hominis was decreased by Halofantrine hydrochloride (adjusted p-values: 7.61E-03). | |||
|
Studied Microbe: Roseburia intestinalis
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Roseburia intestinalis was decreased by Halofantrine hydrochloride (adjusted p-values: 8.94E-04). | |||
|
Studied Microbe: Ruminococcus gnavus
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Ruminococcus gnavus was decreased by Halofantrine hydrochloride (adjusted p-values: 6.93E-03). | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 020250. | |||
| REF 2 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

