Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07NAY
|
|||
| Former ID |
DNC013601
|
|||
| Drug Name |
ROBINETIN
|
|||
| Synonyms |
Robinetin; 490-31-3; Norkanugin; 3,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one; 5-Hydroxyfisetin; 5-Deoxymyricetin; 4H-1-Benzopyran-4-one, 3,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-; UNII-KJ6DBC4U7E; 3,7,3',4',5'-pentahydroxyflavone; NSC 656274; NSC 407331; CCRIS 7520; EINECS 207-709-6; KJ6DBC4U7E; NSC-407331; BRN 0308905; CHEMBL170405; CHEBI:8876; FLAVONE, 3,3',4',5',7-PENTAHYDROXY-; NSC656274; 3,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)-4-benzopyrone
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
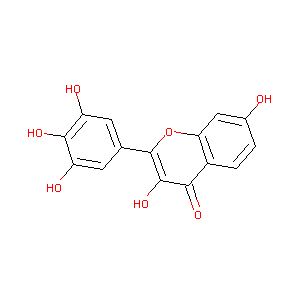 |
Download2D MOL |
||
| Formula |
C15H10O7
|
|||
| Canonical SMILES |
C1=CC2=C(C=C1O)OC(=C(C2=O)O)C3=CC(=C(C(=C3)O)O)O
|
|||
| InChI |
1S/C15H10O7/c16-7-1-2-8-11(5-7)22-15(14(21)12(8)19)6-3-9(17)13(20)10(18)4-6/h1-5,16-18,20-21H
|
|||
| InChIKey |
SOEDEYVDCDYMMH-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 490-31-3
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:8876
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Xanthine dehydrogenase/oxidase (XDH) | Target Info | Inhibitor | [1] |
| BioCyc | Purine nucleotides degradation | |||
| Urate biosynthesis/inosine 5'-phosphate degradation | ||||
| Guanosine nucleotides degradation | ||||
| Adenosine nucleotides degradation | ||||
| Retinoate biosynthesis II | ||||
| KEGG Pathway | Purine metabolism | |||
| Caffeine metabolism | ||||
| Drug metabolism - other enzymes | ||||
| Metabolic pathways | ||||
| Peroxisome | ||||
| Panther Pathway | Adenine and hypoxanthine salvage pathway | |||
| Purine metabolism | ||||
| Pathwhiz Pathway | Caffeine Metabolism | |||
| Purine Metabolism | ||||
| Reactome | Purine catabolism | |||
| WikiPathways | Oxidative Stress | |||
| Effects of Nitric Oxide | ||||
| Metabolism of nucleotides | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Inhibition of cow's milk xanthine oxidase by flavonoids. J Nat Prod. 1988 Mar-Apr;51(2):345-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

