Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08UUL
|
|||
| Drug Name |
LY2606368
|
|||
| Synonyms |
prexasertib; Prexasertib; 1234015-52-1; UNII-820NH671E6; LY-2606368; 820NH671E6; Prexasertib [USAN]; 5-((5-(2-(3-aminopropoxy)-6-methoxyphenyl)-1H-pyrazol-3-yl)amino)pyrazine-2-carbonitrile; 5-({5-[2-(3-aminopropoxy)-6-methoxyphenyl]-1H-pyrazol-3-yl}amino)pyrazine-2-carbonitrile; 5-[[5-[2-(3-aminopropoxy)-6-methoxyphenyl]-1H-pyrazol-3-yl]amino]pyrazine-2-carbonitrile; SCHEMBL1975451; GTPL9549; SCHEMBL19457660; SCHEMBL18989301; CHEMBL3544911; EX-A758; DOTGPNHGTYJDEP-UHFFFAOYSA-N; AOB87325; ZINC95837013
Click to Show/Hide
|
|||
| Indication | Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Phase 2 | [1] | |
| Small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162.9] | Phase 2 | [1] | ||
| Head and neck cancer [ICD-11: 2D42] | Phase 1 | [1] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 1 | [2] | ||
| Squamous cell anal carcinoma [ICD-11: 2C00.3; ICD-9: 154.2, 154.3] | Phase 1 | [3] | ||
| Company |
Eli Lilly, Indianapolis, IN
|
|||
| Structure |
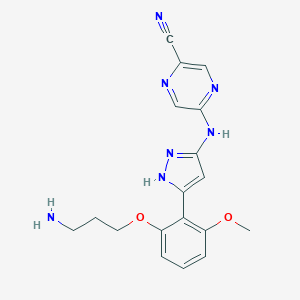 |
Download2D MOL |
||
| Formula |
C18H19N7O2
|
|||
| Canonical SMILES |
COC1=C(C(=CC=C1)OCCCN)C2=CC(=NN2)NC3=NC=C(N=C3)C#N
|
|||
| InChI |
1S/C18H19N7O2/c1-26-14-4-2-5-15(27-7-3-6-19)18(14)13-8-16(25-24-13)23-17-11-21-12(9-20)10-22-17/h2,4-5,8,10-11H,3,6-7,19H2,1H3,(H2,22,23,24,25)
|
|||
| InChIKey |
DOTGPNHGTYJDEP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1234015-52-1
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of Eli Lilly. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

