Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A5EF
|
|||
| Former ID |
DCL000831
|
|||
| Drug Name |
GW-427353
|
|||
| Synonyms |
UNII-GU14FR8D4A; GU14FR8D4A; GW427353B; Solabegron hydrochloride [USAN]; Solabegron hydrochloride (USAN); GW 427353B; Solabegron Hydrochloride; Solabegron HCl; SCHEMBL565177; CHEMBL2107299; PMXCGBVBIRYFPR-FTBISJDPSA-N; BCP28076; 3'-[(2-{[2-(3-chlorophenyl)-2-hydroxyethyl]amino}ethyl)amino]biphenyl-3-carboxylic acid hydrochloride; D05879; (1,1'-Biphenyl)-3-carboxylic acid, 3'-((2-(((2R)-2-(3-chlorophenyl)-2-hydroxyethyl)amino)ethyl)amino)-, hydrochloride
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Urinary incontinence [ICD-11: MF50.2; ICD-9: 788.3] | Phase 2 | [1] | |
| Company |
GSK
|
|||
| Structure |
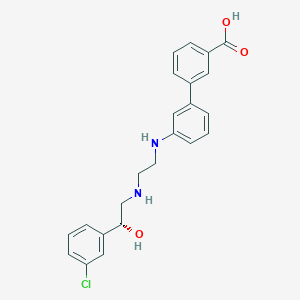 |
Download2D MOL |
||
| Formula |
C23H23ClN2O3
|
|||
| Canonical SMILES |
C1=CC(=CC(=C1)C(=O)O)C2=CC(=CC=C2)NCCNCC(C3=CC(=CC=C3)Cl)O
|
|||
| InChI |
1S/C23H23ClN2O3/c24-20-8-2-6-18(13-20)22(27)15-25-10-11-26-21-9-3-5-17(14-21)16-4-1-7-19(12-16)23(28)29/h1-9,12-14,22,25-27H,10-11,15H2,(H,28,29)/t22-/m0/s1
|
|||
| InChIKey |
LLDXOPKUNJTIRF-QFIPXVFZSA-N
|
|||
| CAS Number |
CAS 252920-94-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:141346
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor beta-3 (ADRB3) | Target Info | Agonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Endocytosis | ||||
| Salivary secretion | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Beta3 adrenergic receptor signaling pathway | ||||
| Reactome | Adrenoceptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00394186) A Study To Investigate GW427353 In Subjects With Irritable Bowel Syndrome (IBS). U.S. National Institutes of Health. | |||
| REF 2 | Emerging drugs for obesity: linking novel biological mechanisms to pharmaceutical pipelines. Expert Opin Emerg Drugs. 2005 Aug;10(3):643-60. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

