Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T51408
(Former ID: TTDC00184)
|
|||||
| Target Name |
Adrenergic receptor beta-3 (ADRB3)
|
|||||
| Synonyms |
Beta3AR; Beta3-AR; Beta-3 adrenoreceptor; Beta-3 adrenoceptor; Beta-3 adrenergic receptor; B3AR; ADRB3R
Click to Show/Hide
|
|||||
| Gene Name |
ADRB3
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 7 Target-related Diseases | + | ||||
| 1 | Angina pectoris [ICD-11: BA40] | |||||
| 2 | Asthma [ICD-11: CA23] | |||||
| 3 | Chronic obstructive pulmonary disease [ICD-11: CA22] | |||||
| 4 | Dystonic disorder [ICD-11: 8A02] | |||||
| 5 | Functional bladder disorder [ICD-11: GC50] | |||||
| 6 | Hypertension [ICD-11: BA00-BA04] | |||||
| 7 | Parkinsonism [ICD-11: 8A00] | |||||
| Function |
Beta-3 is involved in the regulation of lipolysis and thermogenesis. Beta-adrenergic receptors mediate the catecholamine-induced activation of adenylate cyclase through the action of G proteins.
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MAPWPHENSSLAPWPDLPTLAPNTANTSGLPGVPWEAALAGALLALAVLATVGGNLLVIV
AIAWTPRLQTMTNVFVTSLAAADLVMGLLVVPPAATLALTGHWPLGATGCELWTSVDVLC VTASIETLCALAVDRYLAVTNPLRYGALVTKRCARTAVVLVWVVSAAVSFAPIMSQWWRV GADAEAQRCHSNPRCCAFASNMPYVLLSSSVSFYLPLLVMLFVYARVFVVATRQLRLLRG ELGRFPPEESPPAPSRSLAPAPVGTCAPPEGVPACGRRPARLLPLREHRALCTLGLIMGT FTLCWLPFFLANVLRALGGPSLVPGPAFLALNWLGYANSAFNPLIYCRSPDFRSAFRRLL CRCGRRLPPEPCAAARPALFPSGVPAARSSPAQPRLCQRLDGASWGVS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T79O0V | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 9 Approved Drugs | + | ||||
| 1 | Amosulalol | Drug Info | Approved | Hypertension | [2] | |
| 2 | Bitolterol | Drug Info | Approved | Chronic obstructive pulmonary disease | [3] | |
| 3 | Bopindolol | Drug Info | Approved | Hypertension | [3] | |
| 4 | Mepindolol | Drug Info | Approved | Angina pectoris | [3] | |
| 5 | Mirabegron | Drug Info | Approved | Overactive bladder | [4], [5] | |
| 6 | Nipradilol | Drug Info | Approved | Angina pectoris | [3] | |
| 7 | Rimiterol | Drug Info | Approved | Asthma | [3] | |
| 8 | Trihexyphenidyl | Drug Info | Approved | Dystonia | [3], [6], [7] | |
| 9 | Vibegron | Drug Info | Approved | Overactive bladder | [8] | |
| Clinical Trial Drug(s) | [+] 18 Clinical Trial Drugs | + | ||||
| 1 | ONO-2506 | Drug Info | Phase 2/3 | Stroke | [9] | |
| 2 | APD418 | Drug Info | Phase 2 | Heart failure with reduced ejection fraction | [10] | |
| 3 | ASP-3652 | Drug Info | Phase 2 | Overactive bladder | [11] | |
| 4 | AZ-40140 | Drug Info | Phase 2 | Type-2 diabetes | [12] | |
| 5 | CL-316,243 | Drug Info | Phase 2 | Obesity | [13], [14] | |
| 6 | CP-331684 | Drug Info | Phase 2 | Diabetic complication | [15] | |
| 7 | GW-427353 | Drug Info | Phase 2 | Urinary incontinence | [16] | |
| 8 | LY-362884 | Drug Info | Phase 2 | Type-2 diabetes | [12] | |
| 9 | LY-377604 | Drug Info | Phase 2 | Obesity | [17] | |
| 10 | N-5984 | Drug Info | Phase 2 | Type-2 diabetes | [12] | |
| 11 | YM-430 | Drug Info | Phase 2 | Hypertension | [18] | |
| 12 | ZD2079 | Drug Info | Phase 2 | Diabetic complication | [19] | |
| 13 | QLT-091568 | Drug Info | Phase 1/2 | Glaucoma/ocular hypertension | [20] | |
| 14 | BMS-196085 | Drug Info | Phase 1 | Diabetic complication | [21] | |
| 15 | KUC-7483 | Drug Info | Phase 1 | Overactive bladder | [22] | |
| 16 | KUL-7211 | Drug Info | Phase 1 | Neurogenic bladder dysfunction | [23] | |
| 17 | Laevo-Bambuterol | Drug Info | Phase 1 | Asthma | [24] | |
| 18 | ZD7114 | Drug Info | Phase 1 | Diabetic complication | [19] | |
| Discontinued Drug(s) | [+] 20 Discontinued Drugs | + | ||||
| 1 | Epanolol | Drug Info | Discontinued in Preregistration | Angina pectoris | [25] | |
| 2 | PW-2101 | Drug Info | Discontinued in Preregistration | Angina pectoris | [26] | |
| 3 | Amibegron | Drug Info | Discontinued in Phase 3 | Mood disorder | [27], [28] | |
| 4 | Adaprolol maleate-SME | Drug Info | Discontinued in Phase 2 | Glaucoma/ocular hypertension | [29] | |
| 5 | ALPRENOXIME HYDROCHLORIDE | Drug Info | Discontinued in Phase 2 | Glaucoma/ocular hypertension | [30] | |
| 6 | OBERADILOL MONOETHYL MALEATE | Drug Info | Discontinued in Phase 2 | Hypertension | [31] | |
| 7 | PROXODOLOL | Drug Info | Discontinued in Phase 2 | Glaucoma/ocular hypertension | [32] | |
| 8 | Rafabegron | Drug Info | Discontinued in Phase 2 | Type-2 diabetes | [33] | |
| 9 | Tienoxolol | Drug Info | Discontinued in Phase 2 | Hypertension | [34] | |
| 10 | NCX 950 | Drug Info | Discontinued in Phase 1/2 | Asthma | [35] | |
| 11 | MN-246 | Drug Info | Discontinued in Phase 1 | Urinary incontinence | [36] | |
| 12 | PAFENOLOL | Drug Info | Discontinued in Phase 1 | Hypertension | [37] | |
| 13 | RO-16-8714 | Drug Info | Discontinued in Phase 1 | Diabetic complication | [38] | |
| 14 | SB 418790 | Drug Info | Discontinued in Phase 1 | Type-2 diabetes | [39] | |
| 15 | BMS-210285 | Drug Info | Terminated | Type-2 diabetes | [41] | |
| 16 | BRL 26830A | Drug Info | Terminated | Obesity | [42] | |
| 17 | H-216/44 | Drug Info | Terminated | Glaucoma/ocular hypertension | [43] | |
| 18 | SM 11044 | Drug Info | Terminated | Asthma | [44] | |
| 19 | SR-58878 | Drug Info | Terminated | Irritable bowel syndrome | [45] | |
| 20 | SR-58894A | Drug Info | Terminated | Gastrointestinal disease | [46] | |
| Preclinical Drug(s) | [+] 5 Preclinical Drugs | + | ||||
| 1 | CL-314698 | Drug Info | Preclinical | Type-2 diabetes | [12] | |
| 2 | CP-114271 | Drug Info | Preclinical | Obesity | [12] | |
| 3 | GCR-1087 | Drug Info | Preclinical | Type-2 diabetes | [12] | |
| 4 | L-742791 | Drug Info | Preclinical | Obesity | [40], [12] | |
| 5 | L-751250 | Drug Info | Preclinical | Obesity | [12] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Modulator | [+] 23 Modulator drugs | + | ||||
| 1 | Amosulalol | Drug Info | [2], [3] | |||
| 2 | Bitolterol | Drug Info | [3], [47] | |||
| 3 | Bopindolol | Drug Info | [3], [48] | |||
| 4 | Mepindolol | Drug Info | [3], [49], [50] | |||
| 5 | Mirabegron | Drug Info | [5] | |||
| 6 | Nipradilol | Drug Info | [51], [3] | |||
| 7 | Rimiterol | Drug Info | [1], [3] | |||
| 8 | YM-430 | Drug Info | [18] | |||
| 9 | KUL-7211 | Drug Info | [64], [65] | |||
| 10 | Laevo-Bambuterol | Drug Info | [66] | |||
| 11 | Epanolol | Drug Info | [67] | |||
| 12 | Adaprolol maleate-SME | Drug Info | [69] | |||
| 13 | ALPRENOXIME HYDROCHLORIDE | Drug Info | [70] | |||
| 14 | OBERADILOL MONOETHYL MALEATE | Drug Info | [71] | |||
| 15 | PROXODOLOL | Drug Info | [72] | |||
| 16 | Rafabegron | Drug Info | [73] | |||
| 17 | Tienoxolol | Drug Info | [74] | |||
| 18 | PAFENOLOL | Drug Info | [77] | |||
| 19 | RO-16-8714 | Drug Info | [78] | |||
| 20 | H-216/44 | Drug Info | [83] | |||
| 21 | SR-58878 | Drug Info | [84] | |||
| 22 | SR-58894A | Drug Info | [85] | |||
| 23 | ER-23006 | Drug Info | [66] | |||
| Agonist | [+] 40 Agonist drugs | + | ||||
| 1 | Trihexyphenidyl | Drug Info | [52] | |||
| 2 | Vibegron | Drug Info | [8] | |||
| 3 | ONO-2506 | Drug Info | [53] | |||
| 4 | ASP-3652 | Drug Info | [55] | |||
| 5 | AZ-40140 | Drug Info | [12] | |||
| 6 | CL-316,243 | Drug Info | [56] | |||
| 7 | CP-331684 | Drug Info | [57] | |||
| 8 | GW-427353 | Drug Info | [12] | |||
| 9 | LY-362884 | Drug Info | [12] | |||
| 10 | LY-377604 | Drug Info | [58] | |||
| 11 | N-5984 | Drug Info | [12] | |||
| 12 | ZD2079 | Drug Info | [59] | |||
| 13 | KUC-7483 | Drug Info | [62], [63] | |||
| 14 | ZD7114 | Drug Info | [59] | |||
| 15 | Amibegron | Drug Info | [28] | |||
| 16 | NCX 950 | Drug Info | [75] | |||
| 17 | MN-246 | Drug Info | [76] | |||
| 18 | SB 418790 | Drug Info | [79] | |||
| 19 | CL-314698 | Drug Info | [12] | |||
| 20 | CP-114271 | Drug Info | [12] | |||
| 21 | GCR-1087 | Drug Info | [12] | |||
| 22 | L-742791 | Drug Info | [12] | |||
| 23 | L-751250 | Drug Info | [12] | |||
| 24 | BMS-210285 | Drug Info | [80] | |||
| 25 | BRL 26830A | Drug Info | [81] | |||
| 26 | BRL 37344 | Drug Info | [82] | |||
| 27 | SM 11044 | Drug Info | [82] | |||
| 28 | (-)-Ro 363 | Drug Info | [86] | |||
| 29 | Carazolol | Drug Info | [89] | |||
| 30 | FMP-825 | Drug Info | [84] | |||
| 31 | prenalterol | Drug Info | [93] | |||
| 32 | SB251023 | Drug Info | [94] | |||
| 33 | T-0509 | Drug Info | [95] | |||
| 34 | Trecadrine | Drug Info | [96] | |||
| 35 | Trimetoquinol | Drug Info | [97] | |||
| 36 | xamoterol | Drug Info | [98] | |||
| 37 | zinterol | Drug Info | [99] | |||
| 38 | [125I]ICYP | Drug Info | [100] | |||
| 39 | [3H](-)CGP 12177 | Drug Info | [101] | |||
| 40 | [3H]CGP12177 | Drug Info | [82] | |||
| Antagonist | [+] 10 Antagonist drugs | + | ||||
| 1 | APD418 | Drug Info | [54] | |||
| 2 | QLT-091568 | Drug Info | [60] | |||
| 3 | PW-2101 | Drug Info | [68] | |||
| 4 | cicloprolol | Drug Info | [90] | |||
| 5 | H87/07 | Drug Info | [90] | |||
| 6 | iodocyanopindolol | Drug Info | [91] | |||
| 7 | L-748337 | Drug Info | [92] | |||
| 8 | L748328 | Drug Info | [92] | |||
| 9 | LK 204-545 | Drug Info | [90] | |||
| 10 | NIHP | Drug Info | [90] | |||
| Inhibitor | [+] 4 Inhibitor drugs | + | ||||
| 1 | BMS-196085 | Drug Info | [61] | |||
| 2 | 1-(1H-Indol-4-yloxy)-3-phenethylamino-propan-2-ol | Drug Info | [87] | |||
| 3 | 1-(2-allylphenoxy)-3-morpholinopropan-2-ol | Drug Info | [88] | |||
| 4 | 1-(2-isopropylphenoxy)-3-morpholinopropan-2-ol | Drug Info | [88] | |||
| Blocker | [+] 1 Blocker drugs | + | ||||
| 1 | MystiLol | Drug Info | [66] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Similarity Proteins
Human Pathway Affiliation
|
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
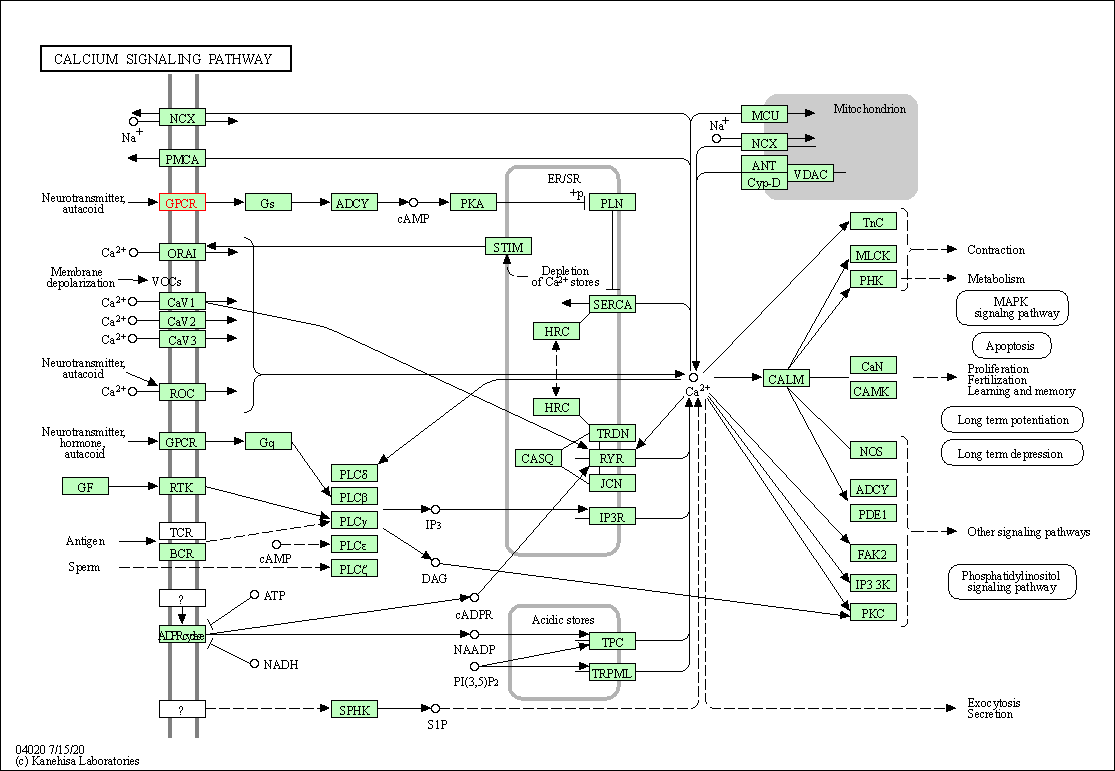
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
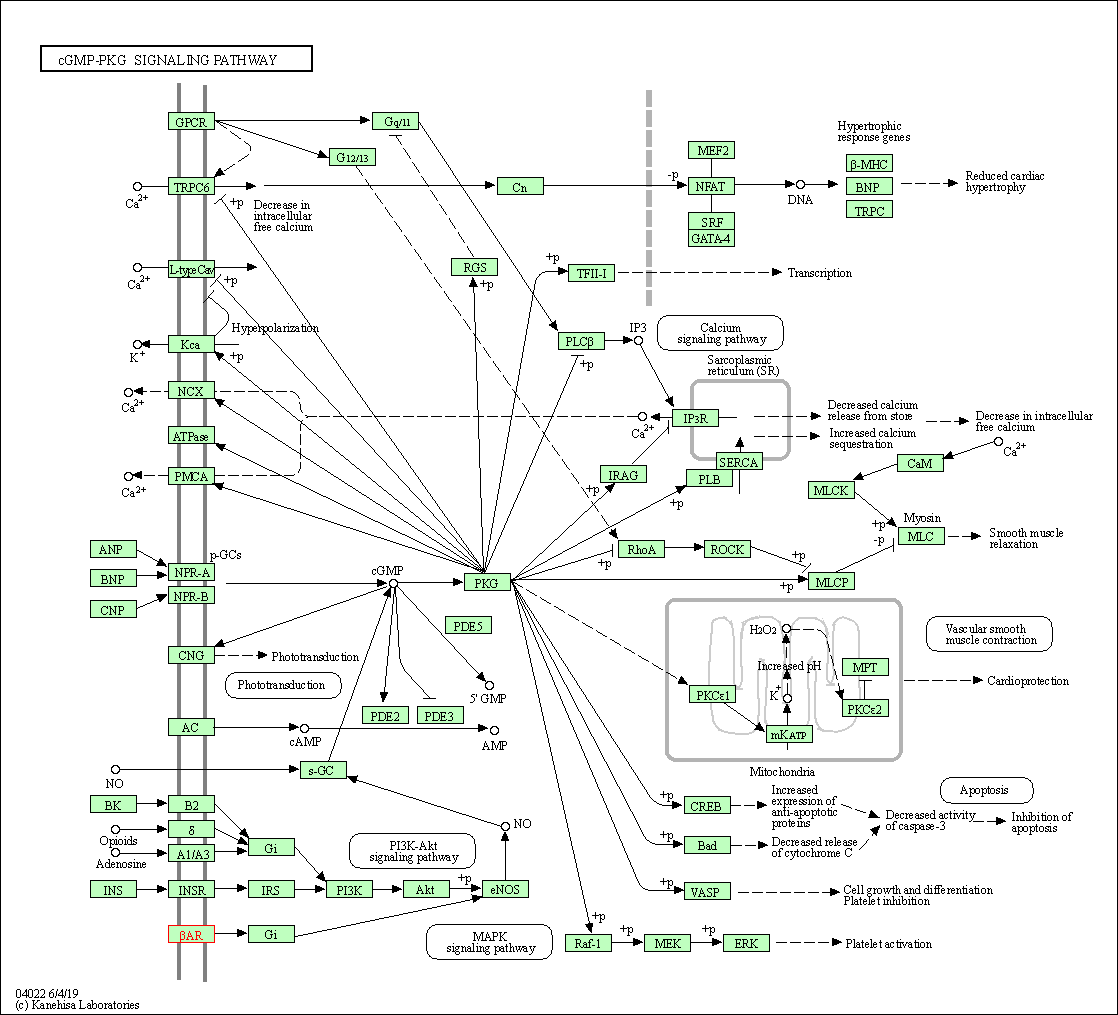
| cGMP-PKG signaling pathway | hsa04022 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
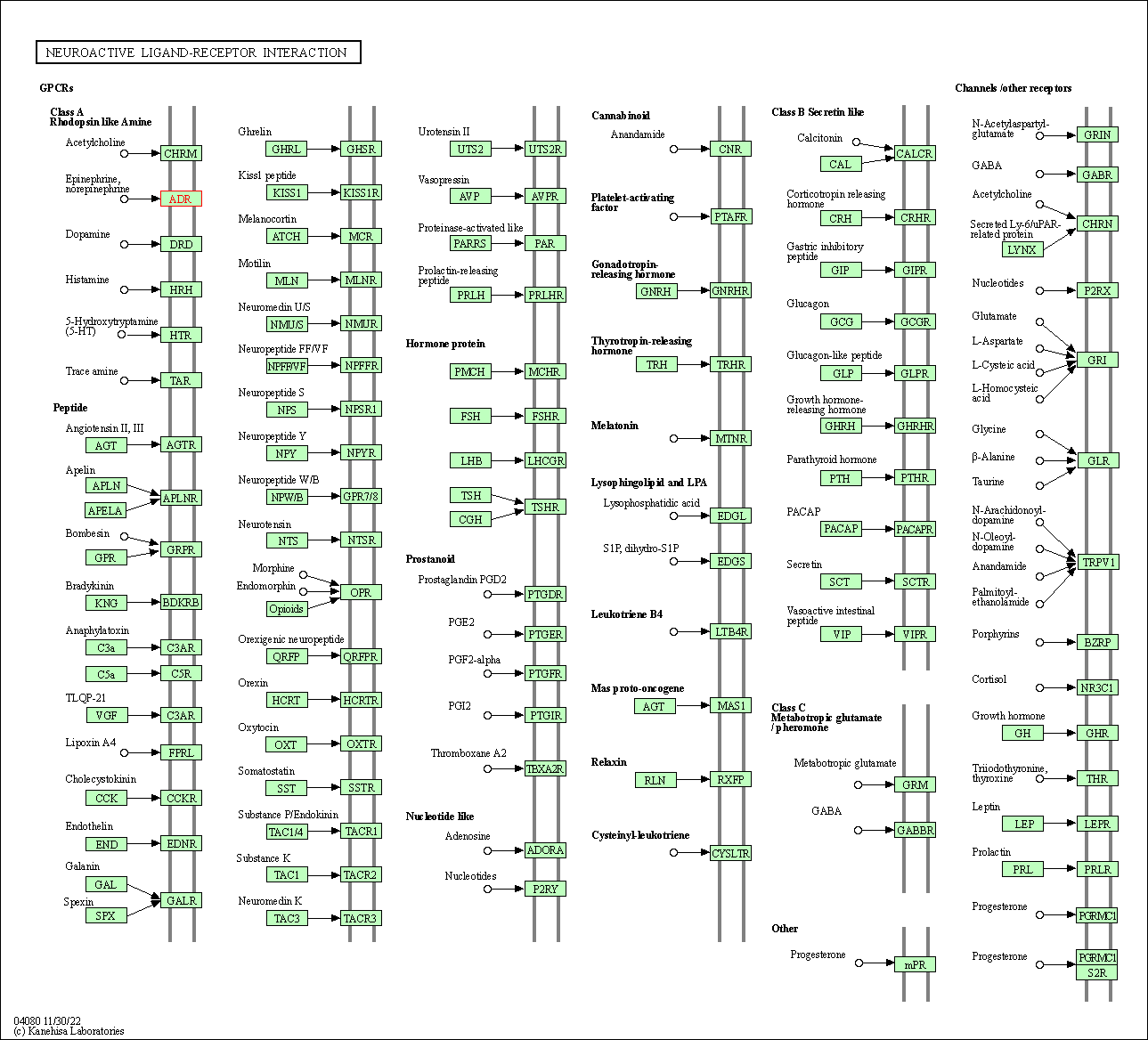
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
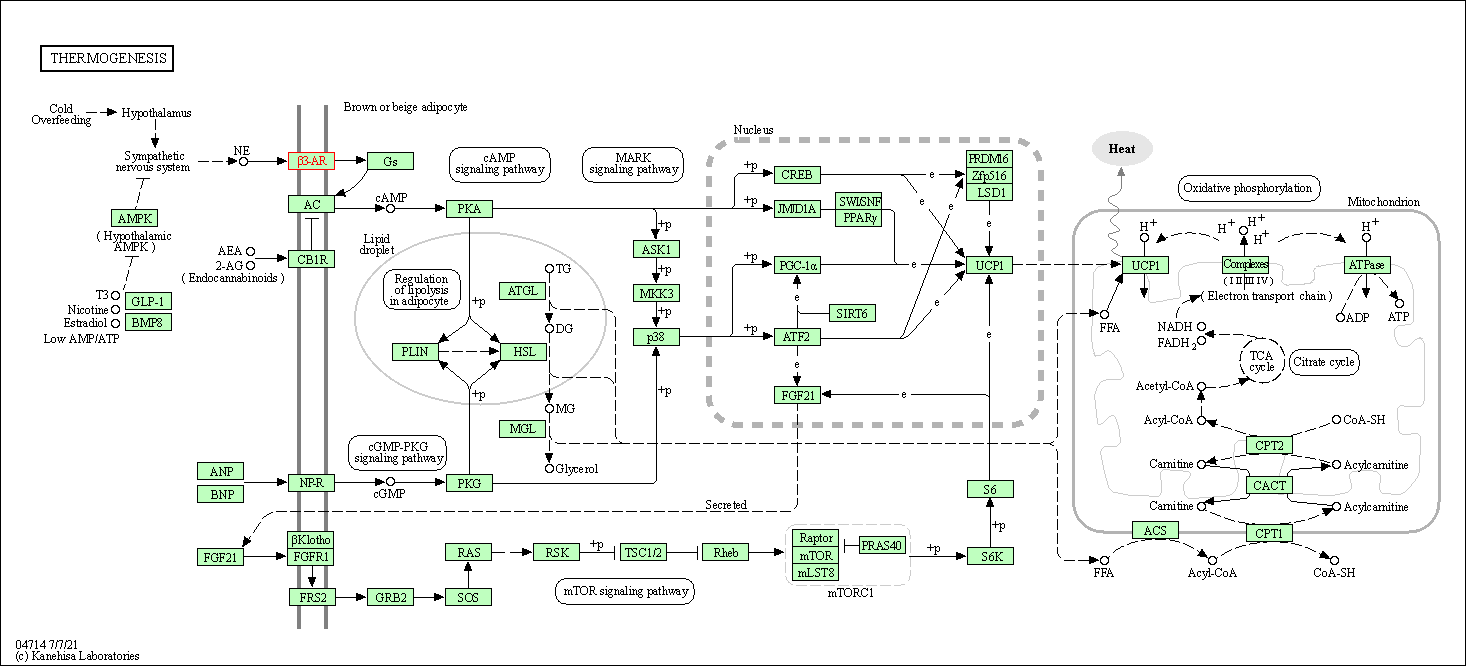
| Thermogenesis | hsa04714 | Affiliated Target |

|
| Class: Organismal Systems => Environmental adaptation | Pathway Hierarchy | ||
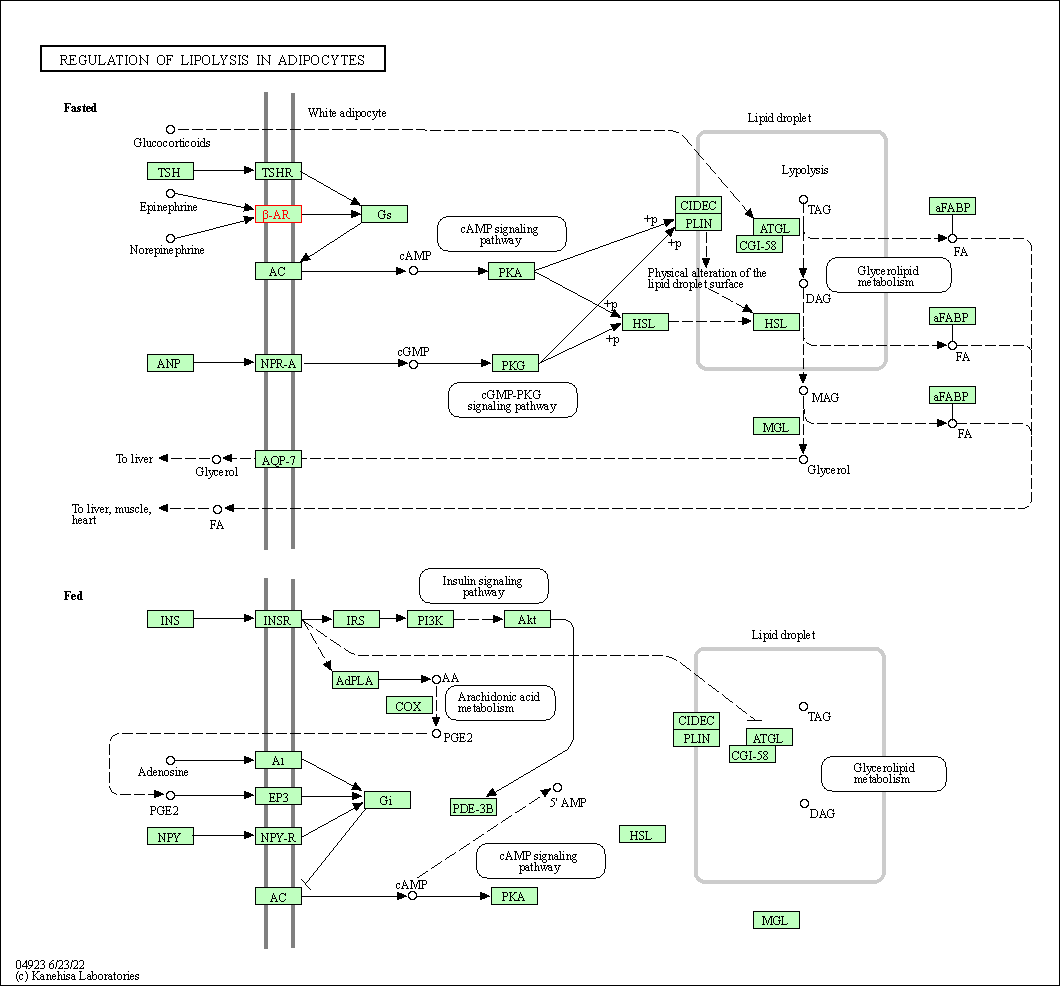
| Regulation of lipolysis in adipocytes | hsa04923 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
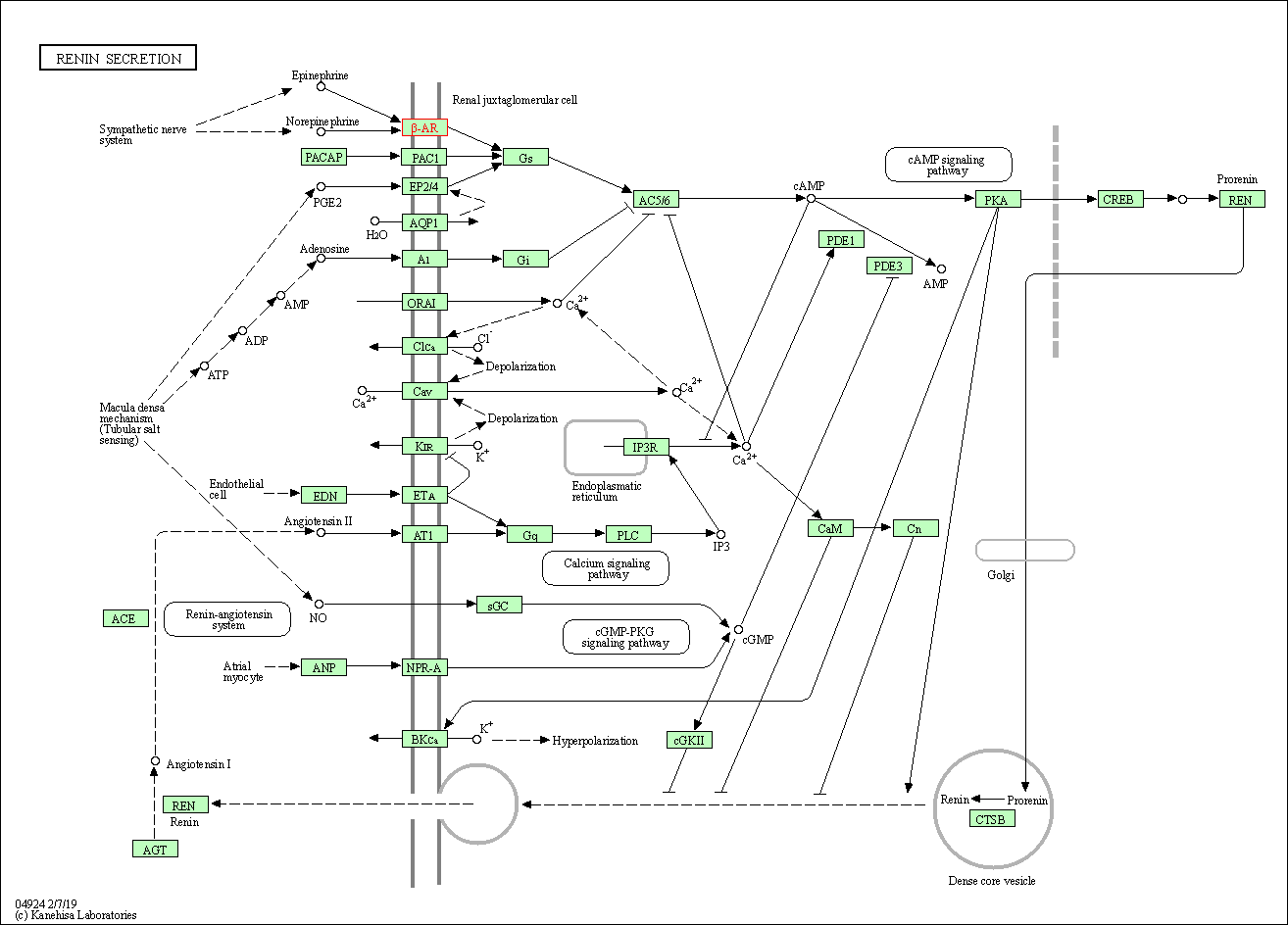
| Renin secretion | hsa04924 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
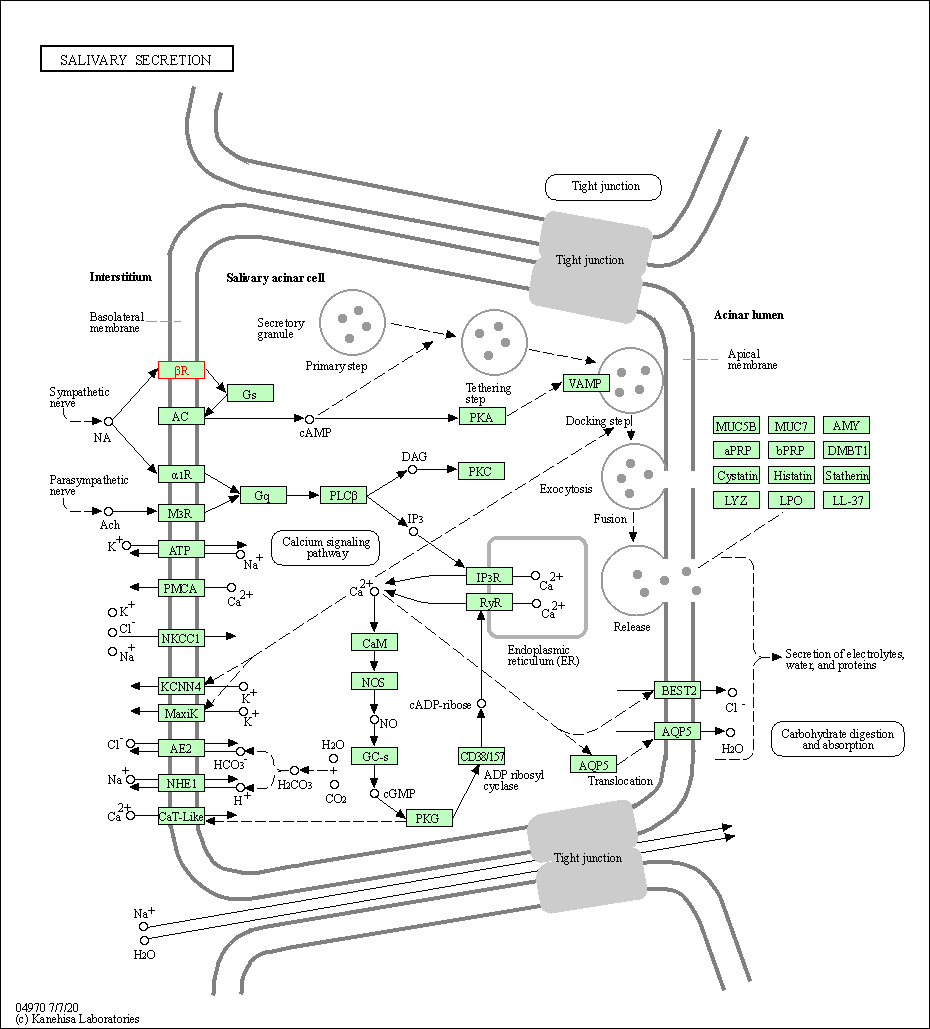
| Salivary secretion | hsa04970 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Drug Resistance Mutation (DRM) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 5 KEGG Pathways | + | ||||
| 1 | Calcium signaling pathway | |||||
| 2 | cGMP-PKG signaling pathway | |||||
| 3 | Neuroactive ligand-receptor interaction | |||||
| 4 | Endocytosis | |||||
| 5 | Salivary secretion | |||||
| Panther Pathway | [+] 2 Panther Pathways | + | ||||
| 1 | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||||
| 2 | Beta3 adrenergic receptor signaling pathway | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Adrenoceptors | |||||
| 2 | G alpha (s) signalling events | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | Monoamine GPCRs | |||||
| 2 | Calcium Regulation in the Cardiac Cell | |||||
| 3 | GPCRs, Class A Rhodopsin-like | |||||
| 4 | GPCR ligand binding | |||||
| 5 | GPCR downstream signaling | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Comparison of the beta2-adrenoceptor selectivity of rimiterol, salbutamol and isoprenaline by the intravenous route in man. Br J Clin Pharmacol. 1975 Feb;2(1):41-8. | |||||
| REF 2 | Effects of amosulalol, a combined alpha 1- and beta-adrenoceptor-blocking agent, on ischemic myocardial energy metabolism in dogs. J Pharm Sci. 1993 Mar;82(3):291-5. | |||||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7445). | |||||
| REF 5 | Nat Rev Drug Discov. 2013 Feb;12(2):87-90. | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7315). | |||||
| REF 7 | Drugs used to treat Parkinson's disease, present status and future directions. CNS Neurol Disord Drug Targets. 2008 Oct;7(4):321-42. | |||||
| REF 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||||
| REF 9 | ClinicalTrials.gov (NCT00229177) Study of ONO-2506 in Patients With Acute Ischemic Stroke. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT05139615) A Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Hemodynamic Effects, Safety, Tolerability, and Pharmacokinetics of APD418 in Subjects With Heart Failure With Reduced Ejection Fraction. U.S.National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT01613586) A Randomized Study Comparing Placebo and ASP3652 in the Treatment of Women With Bladder Pain Syndrome / Interstitial Cystitis (BPS/IC). U.S. National Institutes of Health. | |||||
| REF 12 | Emerging drugs for obesity: linking novel biological mechanisms to pharmaceutical pipelines. Expert Opin Emerg Drugs. 2005 Aug;10(3):643-60. | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3462). | |||||
| REF 14 | Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994 Apr;266(4 Pt 2):R1371-82. | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013231) | |||||
| REF 16 | ClinicalTrials.gov (NCT00394186) A Study To Investigate GW427353 In Subjects With Irritable Bowel Syndrome (IBS). U.S. National Institutes of Health. | |||||
| REF 17 | ClinicalTrials.gov (NCT00993421) A Weight Loss Study in Overweight Men and Women. U.S. National Institutes of Health. | |||||
| REF 18 | Cardiovascular effects of YM430, a 1,4-dihydropyridine derivative with beta-adrenoceptor blocking activity, in dogs and rats. Biol Pharm Bull. 1997 Mar;20(3):230-6. | |||||
| REF 19 | Effects of the two beta3-agonists, ZD7114 and ZD2079 on 24 hour energy expenditure and respiratory quotient in obese subjects. Int J Obes Relat Metab Disord. 2000 Dec;24(12):1553-60. | |||||
| REF 20 | ClinicalTrials.gov (NCT00753168) Phase 1-2 Evaluation of OT-730 Eye Drops in Reducing the Intraocular Pressure in Patients With Ocular Hypertension or Open-Angle Glaucoma. U.S. National Institutes ofHealth. | |||||
| REF 21 | BMS-196085: a potent and selective full agonist of the human beta(3) adrenergic receptor. Bioorg Med Chem Lett. 2001 Dec 3;11(23):3041-4. | |||||
| REF 22 | ClinicalTrials.gov (NCT02256735) Study to Investigate the Effect of KUC 7483 CL on the QT/QTc Interval of the ECG in Comparison to Placebo and Moxifloxacin in Healthy Male and Female Volunteers. U.S.National Institutes of Health. | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013676) | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031995) | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000427) | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019361) | |||||
| REF 27 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 568). | |||||
| REF 28 | Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008 Aug;33(9):2080-92. | |||||
| REF 29 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003472) | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003470) | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002312) | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009043) | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009164) | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000171) | |||||
| REF 35 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012159) | |||||
| REF 36 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022773) | |||||
| REF 37 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002399) | |||||
| REF 38 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001618) | |||||
| REF 39 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013384) | |||||
| REF 40 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3467). | |||||
| REF 41 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010623) | |||||
| REF 42 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000393) | |||||
| REF 43 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005323) | |||||
| REF 44 | The iodocyanopindolol and SM-11044 binding protein belongs to the TM9SF multispanning membrane protein superfamily. Gene. 2001 Aug 8;273(2):227-37. | |||||
| REF 45 | Clinical pipeline report, company report or official report of Sanofi-Synthelabo. | |||||
| REF 46 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008218) | |||||
| REF 47 | Bitolterol mesylate: a beta-adrenergic agent. Chemistry, pharmacokinetics, pharmacodynamics, adverse effects and clinical efficacy in asthma. Pharmacotherapy. 1985 May-Jun;5(3):127-37. | |||||
| REF 48 | Effect of propranolol, alprenolol, pindolol, and bopindolol on beta 2-adrenoceptor density in human lymphocytes. J Cardiovasc Pharmacol. 1986;8 Suppl 6:S70-3. | |||||
| REF 49 | Pharmacological studies on the intrinsic sympathomimetic activity of the beta-adrenoceptor antagonist mepindolol. Arzneimittelforschung. 1986 May;36(5):811-3. | |||||
| REF 50 | Pindolol--a beta-adrenoceptor blocking drug with partial agonist activity: clinical pharmacological considerations.. Br J Clin Pharmacol. 1982; 13(Suppl 2): 187S-192S. | |||||
| REF 51 | Actions of nipradilol (K-351), a new alpha- and beta-adrenoceptor blocker, on the rabbit portal vein. Jpn J Pharmacol. 1984 Aug;35(4):359-69. | |||||
| REF 52 | Obesity: pathophysiology and clinical management. Curr Med Chem. 2009;16(4):506-21. | |||||
| REF 53 | FR149175, a beta 3-adrenoceptor-selective agonist, is a possible therapeutic agent for non-insulin-dependent diabetes mellitus. Jpn J Pharmacol. 1997 May;74(1):109-12. | |||||
| REF 54 | Intravenous Infusion of the beta(3)-Adrenergic Receptor Antagonist APD418 Improves Left Ventricular Systolic Function in Dogs With Systolic Heart Failure. J Card Fail. 2021 Feb;27(2):242-252. | |||||
| REF 55 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800029610) | |||||
| REF 56 | Beta 3-adrenoceptor agonists as anti-diabetic and anti-obesity drugs in humans. Curr Pharm Des. 2001 Sep;7(14):1433-49. | |||||
| REF 57 | CN patent application no. 1717230, Pharmaceutical composition comprising a beta-3-adrenoceptor agonist and a serotonin and/or norepinephrine reuptake inhibitor. | |||||
| REF 58 | Combination of a Beta Adrenoceptor Modulator and a Norepinephrine-Serotonin Uptake Inhibitor for the Treatment of Obesity. ACS Med Chem Lett. 2011 August 11; 2(8): 583-586. | |||||
| REF 59 | Urinary tract toxicity in rats following administration of beta 3-adrenoceptor agonists. Toxicol Pathol. 1999 Mar-Apr;27(2):165-70. | |||||
| REF 60 | Clinical pipeline report, company report or official report of QLT Inc. | |||||
| REF 61 | Arylpropanolamines: selective beta3 agonists arising from strategies to mitigate phase I metabolic transformations. Bioorg Med Chem Lett. 2007 Aug 1;17(15):4290-6. | |||||
| REF 62 | Effects of ritobegron (KUC-7483), a novel selective beta3-adrenoceptor agonist, on bladder function in cynomolgus monkey. J Pharmacol Exp Ther. 2012 Jul;342(1):163-8. | |||||
| REF 63 | Mirabegron for the treatment of overactive bladder. Drugs Today (Barc). 2012 Jan;48(1):25-32. | |||||
| REF 64 | The potency of KUL-7211, a selective ureteral relaxant, in isolated canine ureter: comparison with various spasmolytics.Urol Res.2005 Dec;33(6):409-14. | |||||
| REF 65 | Pharmacological profile of KUL-7211, a selective beta-adrenoceptor agonist, in isolated ureteral smooth muscle. J Pharmacol Sci. 2003 Aug;92(4):411-9. | |||||
| REF 66 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 29). | |||||
| REF 67 | Pharmacokinetics of epanolol after acute and chronic oral dosing in elderly patients with stable angina pectoris. Br J Clin Pharmacol. 1990 Mar;29(3):333-7. | |||||
| REF 68 | PW 2101 Penwest discontinued, USA (hypertension) Penwest non-approvable, USA (hypertension), R & D Focus Drug News. July 11, 2005 | |||||
| REF 69 | Synthesis and pharmacological activity of adaprolol enantiomers: a new soft drug for treating glaucoma. J Ocul Pharmacol Ther. 1996 Summer;12(2):115-22. | |||||
| REF 70 | Improved delivery through biological membranes. LVI. Pharmacological evaluation of alprenoxime--a new potential antiglaucoma agent. Pharm Res. 1991 Nov;8(11):1389-95. | |||||
| REF 71 | US patent application no. 9,062,094, Dipeptide-based prodrug linkers for aliphatic amine-containing drugs. | |||||
| REF 72 | The efficacy of proxodolol, a new beta-adrenoblockader with alpha-adrenoblockader properties, in its single use in patients with stable stenocardia of effort. Eksp Klin Farmakol. 1994 May-Jun;57(3):47-50. | |||||
| REF 73 | Lack of an effect of a novel beta3-adrenoceptor agonist, TAK-677, on energy metabolism in obese individuals: a double-blind, placebo-controlled ran... J Clin Endocrinol Metab. 2007 Feb;92(2):527-31. | |||||
| REF 74 | Effects of the beta-adrenoceptor antagonists atenolol and propranolol on human parotid and submandibular-sublingual salivary secretion. J Dent Res. 1994 Jan;73(1):5-10. | |||||
| REF 75 | A Nitric Oxide-Releasing Salbutamol Elicits Potent Relaxant and Anti-Inflammatory Activities. JPET July 2004 vol. 310 no. 1 367-375. | |||||
| REF 76 | CA patent application no. 630818, Pharmaceutical composition for prevention or treatment of neurogenic pain. | |||||
| REF 77 | Presystemic elimination of the beta-blocker pafenolol in the rat after oral and intraperitoneal administration and identification of a main metabolite in both rats and humans. Drug Metab Dispos. 1993May-Jun;21(3):435-40. | |||||
| REF 78 | The novel thermogenic beta-adrenergic agonist Ro 16-8714 increases the interscapular brown-fat beta-receptor-adenylate cyclase and the uncoupling-protein mRNA level in obese (fa/fa) Zucker rats. Biochem J. 1989 Aug 1;261(3):721-4. | |||||
| REF 79 | News and Analysis. Nature Reviews Drug Discovery 1, 257-258 (April 2002). | |||||
| REF 80 | CA patent application no. 509835, Nitrogen-containing heterocyclic derivatives, medicinal compositions containing the same and medicinal use thereof. | |||||
| REF 81 | Anti-obesity and anti-diabetic actions of a beta 3-adrenoceptor agonist, BRL 26830A, in yellow KK mice. Endocrinol Jpn. 1991 Aug;38(4):397-403. | |||||
| REF 82 | Effects of several putative beta 3-adrenoceptor agonists on lipolysis in human omental adipocytes. Int J Obes Relat Metab Disord. 1996 May;20(5):428-34. | |||||
| REF 83 | Binding of the beta-blockers timolol and H 216/44 to ocular melanin. Exp Eye Res. 1988 Oct;47(4):565-77. | |||||
| REF 84 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 30). | |||||
| REF 85 | Pharmacological characterization of beta-adrenoceptor subtypes mediating relaxation in porcine isolated ureteral smooth muscle. J Urol. 2004 Sep;172(3):1155-9. | |||||
| REF 86 | Effects of (-)-RO363 at human atrial beta-adrenoceptor subtypes, the human cloned beta 3-adrenoceptor and rodent intestinal beta 3-adrenoceptors. Br J Pharmacol. 1997 Jan;120(2):165-76. | |||||
| REF 87 | Synthesis and beta-adrenergic receptor blocking potency of 1-(substituted amino)-3-(4-indolyloxy)propan-2-ols. J Med Chem. 1986 Aug;29(8):1524-7. | |||||
| REF 88 | A vHTS approach for the identification of beta-adrenoceptor ligands. Bioorg Med Chem Lett. 2010 Jun 1;20(11):3399-404. | |||||
| REF 89 | Current therapeutic uses and potential of beta-adrenoceptor agonists and antagonists. Eur J Clin Pharmacol. 1998 Feb;53(6):389-404. | |||||
| REF 90 | LK 204-545, a highly selective beta1-adrenoceptor antagonist at human beta-adrenoceptors. Eur J Pharmacol. 1999 Feb 19;367(2-3):431-5. | |||||
| REF 91 | Markedly reduced effects of (-)-isoprenaline but not of (-)-CGP12177 and unchanged affinity of beta-blockers at Gly389-beta1-adrenoceptors compared... Br J Pharmacol. 2004 May;142(1):51-6. | |||||
| REF 92 | Potent and selective human beta(3)-adrenergic receptor antagonists. J Pharmacol Exp Ther. 1999 Aug;290(2):649-55. | |||||
| REF 93 | Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol. 1989 Mar;35(3):295-303. | |||||
| REF 94 | Mouse beta 3a- and beta 3b-adrenoceptors expressed in Chinese hamster ovary cells display identical pharmacology but utilize distinct signalling pathways. Br J Pharmacol. 2002 Apr;135(8):1903-14. | |||||
| REF 95 | Molecular characterization of pharmacological properties of T-0509 for beta-adrenoceptors. Eur J Pharmacol. 1996 Nov 21;315(3):363-7. | |||||
| REF 96 | Potential anti-diabetic applications of a new molecule with affinity for beta 3-adrenoceptors. Life Sci. 1996;59(11):PL141-6. | |||||
| REF 97 | Synthesis and human beta-adrenoceptor activity of 1-(3,5-diiodo-4- methoxybenzyl)-1,2,3,4-tetrahydroisoquinolin-6-ol derivatives in vitro. J Med Chem. 2000 Feb 24;43(4):591-8. | |||||
| REF 98 | Binding pockets of the beta(1)- and beta(2)-adrenergic receptors for subtype-selective agonists. Mol Pharmacol. 1999 Nov;56(5):875-85. | |||||
| REF 99 | The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol. 2010 Jul;160(5):1048-61. | |||||
| REF 100 | Stereoselectivity for interactions of agonists and antagonists at mouse, rat and human beta3-adrenoceptors. Eur J Pharmacol. 2004 Jan 26;484(2-3):323-31. | |||||
| REF 101 | Binding of (-)-[3H]-CGP12177 at two sites in recombinant human beta 1-adrenoceptors and interaction with beta-blockers. Naunyn Schmiedebergs Arch Pharmacol. 2004 May;369(5):525-32. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

