Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DFS18G
|
|||
| Drug Name |
APD418
|
|||
| Synonyms |
Vemtoberant; 2169905-68-2; vemtoberant [INN]; 4IJ6WR3BW8; APD418; SCHEMBL19707104; APD-418; BDBM484072; EX-A7995; US10927123, Compound 310; HY-148804; CS-0641193; 1-ethyl-3-((R)-3-((S)-2- hydroxy-3-(3- (methylsulfonyl)phenoxy) propylamino)-1-oxa- 8-azaspiro[4.5]decan-8- ylsulfonyl)quinolin- 4(1H)-one; 1-ethyl-3-[(3R)-3-({(2S)-2-hydroxy-3-[3- (methanesulfonyl)phenoxy]propyl}amino)-1-oxa-8- azaspiro[4.5]decane-8-sulfonyl]quinolin-4(1H)-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Heart failure with reduced ejection fraction [ICD-11: BD11.2] | Phase 2 | [1] | |
| Company |
Pfzer
|
|||
| Structure |
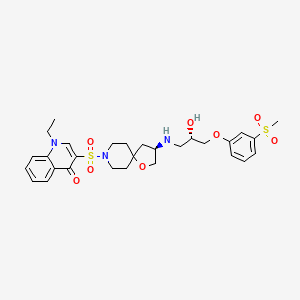 |
Download2D MOL |
||
| Formula |
C29H37N3O8S2
|
|||
| Canonical SMILES |
CCN1C=C(C(=O)C2=CC=CC=C21)S(=O)(=O)N3CCC4(CC3)CC(CO4)NCC(COC5=CC(=CC=C5)S(=O)(=O)C)O
|
|||
| InChI |
InChI=1S/C29H37N3O8S2/c1-3-31-18-27(28(34)25-9-4-5-10-26(25)31)42(37,38)32-13-11-29(12-14-32)16-21(19-40-29)30-17-22(33)20-39-23-7-6-8-24(15-23)41(2,35)36/h4-10,15,18,21-22,30,33H,3,11-14,16-17,19-20H2,1-2H3/t21-,22+/m1/s1
|
|||
| InChIKey |
PVXLIOIISUUKOQ-YADHBBJMSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor beta-3 (ADRB3) | Target Info | Antagonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Endocytosis | ||||
| Salivary secretion | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Beta3 adrenergic receptor signaling pathway | ||||
| Reactome | Adrenoceptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05139615) A Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Hemodynamic Effects, Safety, Tolerability, and Pharmacokinetics of APD418 in Subjects With Heart Failure With Reduced Ejection Fraction. U.S.National Institutes of Health. | |||
| REF 2 | Intravenous Infusion of the beta(3)-Adrenergic Receptor Antagonist APD418 Improves Left Ventricular Systolic Function in Dogs With Systolic Heart Failure. J Card Fail. 2021 Feb;27(2):242-252. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

