Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0BM7M
|
|||
| Former ID |
DCL000486
|
|||
| Drug Name |
AZD8931
|
|||
| Synonyms |
Sapitinib; AZD8931; 848942-61-0; AZD8931 (Sapitinib); AZD-8931; Sapitinib(AZD8931); UNII-3499328002; CHEMBL2408045; 2-(4-((4-((3-Chloro-2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)oxy)piperidin-1-yl)-n-methylacetamide; 2-[4-({4-[(3-chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl}oxy)piperidin-1-yl]-N-methylacetamide; Sapitinib [INN]; 4-(3-chloro-2-fluoroanilino)-7-methoxy-6-((1-(n-methylcarbamoylmethyl)piperidin-4-yl)oxy)quinazoline
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Breast cancer [ICD-11: 2C60-2C65] | Phase 2 | [1], [2] | |
| Company |
AstraZeneca
|
|||
| Structure |
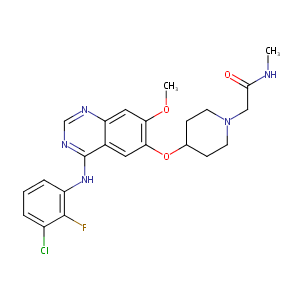 |
Download2D MOL |
||
| Formula |
C23H25ClFN5O3
|
|||
| Canonical SMILES |
CNC(=O)CN1CCC(CC1)OC2=C(C=C3C(=C2)C(=NC=N3)NC4=C(C(=CC=C4)Cl)F)OC
|
|||
| InChI |
1S/C23H25ClFN5O3/c1-26-21(31)12-30-8-6-14(7-9-30)33-20-10-15-18(11-19(20)32-2)27-13-28-23(15)29-17-5-3-4-16(24)22(17)25/h3-5,10-11,13-14H,6-9,12H2,1-2H3,(H,26,31)(H,27,28,29)
|
|||
| InChIKey |
DFJSJLGUIXFDJP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 848942-61-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
16590003, 23622332, 42567199, 80594667, 104253512, 124757635, 125164439, 135727457, 135727830, 136367466, 136368073, 136920286, 137166714, 138013466, 138192963, 144115660, 162011769, 162037806, 163642707, 163908019, 165246681, 174006599, 174507796, 174530908, 175449670, 185992959, 198989379, 223366050, 223401932, 223705284, 226558701, 244694478, 249814500, 251962992, 252214940, 252439600, 252451838
|
|||
| ChEBI ID |
CHEBI:132986
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7717). | |||
| REF 2 | ClinicalTrials.gov (NCT02117167) Intergroup Trial UNICANCER UC 0105-1305/ IFCT 1301: Efficacy of Targeted Drugs Guided by Genomic Profils in Metastatic NSCLC Patients. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||
| REF 4 | Characterization of AZD8931, a potent reversible small molecule inhibitor against epidermal growth factor receptor (EGFR), erythroblastic leukemia viral oncogene homolog 2 (HER2) and 3 (HER3) with a unique and balanced pharmacological profile. J Clin Oncol 27:15s, 2009. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

