Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T14597
(Former ID: TTDS00249)
|
|||||
| Target Name |
Erbb2 tyrosine kinase receptor (HER2)
|
|||||
| Synonyms |
p185erbB2; Tyrosine kinase-type cell surface receptor HER2; Receptor tyrosine-protein kinase erbB-2; Proto-oncogene c-ErbB-2; Proto-oncogene Neu; NGL; NEU; Metastatic lymph node gene 19 protein; MLN19; MLN 19; HER2; CD340
Click to Show/Hide
|
|||||
| Gene Name |
ERBB2
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Breast cancer [ICD-11: 2C60-2C6Y] | |||||
| 2 | Lung cancer [ICD-11: 2C25] | |||||
| 3 | Prostate cancer [ICD-11: 2C82] | |||||
| Function |
Protein tyrosine kinase that is part of several cell surface receptor complexes, but that apparently needs a coreceptor for ligand binding. Essential component of a neuregulin-receptor complex, although neuregulins do not interact with it alone. GP30 is a potential ligand for this receptor. Regulates outgrowth and stabilization of peripheral microtubules (MTs). Upon ERBB2 activation, the MEMO1-RHOA-DIAPH1 signaling pathway elicits the phosphorylation and thus the inhibition of GSK3B at cell membrane. This prevents the phosphorylation of APC and CLASP2, allowing its association with the cell membrane. In turn, membrane-bound APC allows the localization of MACF1 to the cell membrane, which is required for microtubule capture and stabilization.
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.10.1
|
|||||
| Sequence |
MELAALCRWGLLLALLPPGAASTQVCTGTDMKLRLPASPETHLDMLRHLYQGCQVVQGNL
ELTYLPTNASLSFLQDIQEVQGYVLIAHNQVRQVPLQRLRIVRGTQLFEDNYALAVLDNG DPLNNTTPVTGASPGGLRELQLRSLTEILKGGVLIQRNPQLCYQDTILWKDIFHKNNQLA LTLIDTNRSRACHPCSPMCKGSRCWGESSEDCQSLTRTVCAGGCARCKGPLPTDCCHEQC AAGCTGPKHSDCLACLHFNHSGICELHCPALVTYNTDTFESMPNPEGRYTFGASCVTACP YNYLSTDVGSCTLVCPLHNQEVTAEDGTQRCEKCSKPCARVCYGLGMEHLREVRAVTSAN IQEFAGCKKIFGSLAFLPESFDGDPASNTAPLQPEQLQVFETLEEITGYLYISAWPDSLP DLSVFQNLQVIRGRILHNGAYSLTLQGLGISWLGLRSLRELGSGLALIHHNTHLCFVHTV PWDQLFRNPHQALLHTANRPEDECVGEGLACHQLCARGHCWGPGPTQCVNCSQFLRGQEC VEECRVLQGLPREYVNARHCLPCHPECQPQNGSVTCFGPEADQCVACAHYKDPPFCVARC PSGVKPDLSYMPIWKFPDEEGACQPCPINCTHSCVDLDDKGCPAEQRASPLTSIISAVVG ILLVVVLGVVFGILIKRRQQKIRKYTMRRLLQETELVEPLTPSGAMPNQAQMRILKETEL RKVKVLGSGAFGTVYKGIWIPDGENVKIPVAIKVLRENTSPKANKEILDEAYVMAGVGSP YVSRLLGICLTSTVQLVTQLMPYGCLLDHVRENRGRLGSQDLLNWCMQIAKGMSYLEDVR LVHRDLAARNVLVKSPNHVKITDFGLARLLDIDETEYHADGGKVPIKWMALESILRRRFT HQSDVWSYGVTVWELMTFGAKPYDGIPAREIPDLLEKGERLPQPPICTIDVYMIMVKCWM IDSECRPRFRELVSEFSRMARDPQRFVVIQNEDLGPASPLDSTFYRSLLEDDDMGDLVDA EEYLVPQQGFFCPDPAPGAGGMVHHRHRSSSTRSGGGDLTLGLEPSEEEAPRSPLAPSEG AGSDVFDGDLGMGAAKGLQSLPTHDPSPLQRYSEDPTVPLPSETDGYVAPLTCSPQPEYV NQPDVRPQPPSPREGPLPAARPAGATLERPKTLSPGKNGVVKDVFAFGGAVENPEYLTPQ GGAAPQPHPPPAFSPAFDNLYYWDQDPPERGAPPSTFKGTPTAENPEYLGLDVPV Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A00770 ; BADD_A01626 ; BADD_A05064 ; BADD_A05258 ; BADD_A06535 | |||||
| HIT2.0 ID | T38K2W | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 10 Approved Drugs | + | ||||
| 1 | BIBW 2992 | Drug Info | Approved | Non-small-cell lung cancer | [2], [3] | |
| 2 | Dacomitinib | Drug Info | Approved | Non-small-cell lung cancer | [4] | |
| 3 | Lapatinib | Drug Info | Approved | Breast cancer | [5], [6] | |
| 4 | Margetuximab | Drug Info | Approved | HER2-positive breast cancer | [7] | |
| 5 | Masoprocol | Drug Info | Approved | Prostate cancer | [8], [9] | |
| 6 | Merimepodib | Drug Info | Approved | Breast cancer | [10] | |
| 7 | NERATINIB MALEATE | Drug Info | Approved | HER2/NEU overexpressing breast cancer | [11] | |
| 8 | Pertuzumab | Drug Info | Approved | Breast cancer | [12], [13] | |
| 9 | Trastuzumab | Drug Info | Approved | Breast cancer | [9], [14], [15] | |
| 10 | Tucatinib | Drug Info | Approved | HER2-positive breast cancer | [16] | |
| Clinical Trial Drug(s) | [+] 77 Clinical Trial Drugs | + | ||||
| 1 | Bevacizumab + Trastuzumab | Drug Info | Phase 3 | Breast cancer | [17] | |
| 2 | HKI-272 | Drug Info | Phase 3 | Breast cancer | [18], [19] | |
| 3 | Nelipepimut S | Drug Info | Phase 3 | Non-hodgkin lymphoma | [20] | |
| 4 | NeuVax | Drug Info | Phase 3 | Breast cancer | [20] | |
| 5 | PF-05280014 | Drug Info | Phase 3 | Breast cancer | [21] | |
| 6 | SYD985 | Drug Info | Phase 3 | Breast cancer | [22] | |
| 7 | Taxol/Paraplatin/Herceptin | Drug Info | Phase 3 | Breast cancer | [23] | |
| 8 | Trastuzumab-DM1 | Drug Info | Phase 3 | Breast cancer | [24] | |
| 9 | Varlitinib | Drug Info | Phase 2/3 | Metastatic biliary tract neoplasms | [19] | |
| 10 | Anti-CD3 and anti-Her2/neu bispecific antibody-armed activated T cells | Drug Info | Phase 2 | Breast cancer | [25] | |
| 11 | AZD8931 | Drug Info | Phase 2 | Breast cancer | [26], [27] | |
| 12 | BMS-599626 | Drug Info | Phase 2 | Solid tumour/cancer | [28], [29] | |
| 13 | CI-1033 | Drug Info | Phase 2 | Lymphoma | [30], [31] | |
| 14 | CP-724714 | Drug Info | Phase 2 | Lymphoma | [32], [33] | |
| 15 | DN24-02 | Drug Info | Phase 2 | Urogenital cancer | [34] | |
| 16 | Ertumaxomab | Drug Info | Phase 2 | Breast cancer | [35], [36] | |
| 17 | HER-2 Protein AutoVac | Drug Info | Phase 2 | Breast cancer | [37] | |
| 18 | Her2-targeted autologous T-cell therapy | Drug Info | Phase 2 | Glioblastoma multiforme | [38] | |
| 19 | HER2/neu peptide vaccine | Drug Info | Phase 2 | Breast cancer | [39] | |
| 20 | HM-78136B | Drug Info | Phase 2 | Solid tumour/cancer | [40], [41] | |
| 21 | KN026 | Drug Info | Phase 2 | Breast cancer | [42] | |
| 22 | MCLA-128 | Drug Info | Phase 2 | Breast cancer | [19] | |
| 23 | MGAH22 | Drug Info | Phase 2 | Solid tumour/cancer | [43] | |
| 24 | MM-111 | Drug Info | Phase 2 | Gastric adenocarcinoma | [44] | |
| 25 | MRG002 | Drug Info | Phase 2 | Urothelial carcinoma | [45] | |
| 26 | Pazopanib + Tyverb/Tykerb | Drug Info | Phase 2 | Inflammatory breast cancer | [46] | |
| 27 | Tarloxotinib | Drug Info | Phase 2 | Non-small-cell lung cancer | [47] | |
| 28 | Zenocutuzumab | Drug Info | Phase 2 | Pancreatic cancer | [48] | |
| 29 | A166 | Drug Info | Phase 1/2 | Solid tumour/cancer | [49] | |
| 30 | ABY-025 | Drug Info | Phase 1/2 | Bladder cancer | [50] | |
| 31 | AGN-208397 | Drug Info | Phase 1/2 | Retina venous occlusion | [51] | |
| 32 | Anti-HER2 CAR-T | Drug Info | Phase 1/2 | Colorectal cancer | [52] | |
| 33 | AU105 | Drug Info | Phase 1/2 | Recurrent glioblastoma | [19], [53], [54] | |
| 34 | AVX901 | Drug Info | Phase 1/2 | Breast cancer | [53], [55], [56] | |
| 35 | BDTX-189 | Drug Info | Phase 1/2 | Solid tumour/cancer | [57] | |
| 36 | CART-HER-2 | Drug Info | Phase 1/2 | Solid tumour/cancer | [58] | |
| 37 | HER-2-targeting CAR T Cells | Drug Info | Phase 1/2 | Breast cancer | [59] | |
| 38 | ISB 1302 | Drug Info | Phase 1/2 | HER2-positive breast cancer | [60] | |
| 39 | Sym013 | Drug Info | Phase 1/2 | Epithelial ovarian cancer | [19] | |
| 40 | TAS-0728 | Drug Info | Phase 1/2 | Solid tumour/cancer | [61] | |
| 41 | Zenocutuzomab | Drug Info | Phase 1/2 | Solid tumour/cancer | [62] | |
| 42 | S-222611 | Drug Info | Phase 1b | Malignant solid tumour | [63] | |
| 43 | ACE1702 | Drug Info | Phase 1 | Aggressive cancer | [64] | |
| 44 | AIP-303 | Drug Info | Phase 1 | Breast cancer | [65] | |
| 45 | ARRY-380 | Drug Info | Phase 1 | Solid tumour/cancer | [66] | |
| 46 | BAY 2701439 | Drug Info | Phase 1 | Solid tumour/cancer | [67] | |
| 47 | BB-1701 | Drug Info | Phase 1 | Aggressive cancer | [68] | |
| 48 | CAR-T Cells targeting HER2 | Drug Info | Phase 1 | Pancreatic cancer | [69] | |
| 49 | CAR-T cells targeting HER2 | Drug Info | Phase 1 | Solid tumour/cancer | [70] | |
| 50 | Cipatinib | Drug Info | Phase 1 | Solid tumour/cancer | [71] | |
| 51 | CUDC-101 | Drug Info | Phase 1 | Solid tumour/cancer | [72] | |
| 52 | DZD1516 | Drug Info | Phase 1 | HER2-positive breast cancer | [73] | |
| 53 | GBR1302 | Drug Info | Phase 1 | Solid tumour/cancer | [74] | |
| 54 | GQ1001 | Drug Info | Phase 1 | Solid tumour/cancer | [75] | |
| 55 | HER2-CAR T Cells | Drug Info | Phase 1 | Malignant neoplasm | [76] | |
| 56 | HER2-specific CAR T cell | Drug Info | Phase 1 | Atypical teratoid/rhabdoid tumour | [77] | |
| 57 | HER2-specific T cells | Drug Info | Phase 1 | Recurrent glioblastoma | [78] | |
| 58 | HER2p63-71 peptide vaccine | Drug Info | Phase 1 | Solid tumour/cancer | [79] | |
| 59 | JNJ-26483327 | Drug Info | Phase 1 | Solid tumour/cancer | [80] | |
| 60 | M802 | Drug Info | Phase 1 | Solid tumour/cancer | [81] | |
| 61 | MB-103 | Drug Info | Phase 1 | Glioblastoma multiforme | [82] | |
| 62 | MBS301 | Drug Info | Phase 1 | Solid tumour/cancer | [83] | |
| 63 | MEDI4276 | Drug Info | Phase 1 | Solid tumour/cancer | [19], [53] | |
| 64 | MM-302 | Drug Info | Phase 1 | Breast cancer | [84] | |
| 65 | MT-5111 | Drug Info | Phase 1 | Solid tumour/cancer | [85] | |
| 66 | MVA HER-2 AutoVac | Drug Info | Phase 1 | Breast cancer | [86] | |
| 67 | NJH395 | Drug Info | Phase 1 | HER2-positive metastatic breast cancer | [87] | |
| 68 | Recombinant human Erbb3 fragment therapeutic tumor vaccine | Drug Info | Phase 1 | Solid tumour/cancer | [88] | |
| 69 | Runimotamab | Drug Info | Phase 1 | Breast cancer | [89] | |
| 70 | SAR443216 | Drug Info | Phase 1 | Gastric cancer | [90] | |
| 71 | SBT6050 | Drug Info | Phase 1 | Solid tumour/cancer | [91] | |
| 72 | TAK-285 | Drug Info | Phase 1 | Solid tumour/cancer | [92] | |
| 73 | TrasGEX | Drug Info | Phase 1 | Solid tumour/cancer | [93] | |
| 74 | TT16 | Drug Info | Phase 1 | Aggressive cancer | [94] | |
| 75 | VM-206 | Drug Info | Phase 1 | Solid tumour/cancer | [95] | |
| 76 | Zemab | Drug Info | Phase 1 | Solid tumour/cancer | [96] | |
| 77 | ZW49 | Drug Info | Phase 1 | Solid tumour/cancer | [97] | |
| Discontinued Drug(s) | [+] 3 Discontinued Drugs | + | ||||
| 1 | Her-2-Bi-armed ATC | Drug Info | Discontinued in Phase 2 | Breast cancer | [98] | |
| 2 | TAK165 | Drug Info | Discontinued in Phase 1 | Solid tumour/cancer | [99], [100] | |
| 3 | GW-974 | Drug Info | Terminated | Breast cancer | [102] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | 227Th-labelled HER2-TTC | Drug Info | Preclinical | Solid tumour/cancer | [101] | |
| Mode of Action | [+] 7 Modes of Action | + | ||||
| Inhibitor | [+] 39 Inhibitor drugs | + | ||||
| 1 | BIBW 2992 | Drug Info | [103] | |||
| 2 | Lapatinib | Drug Info | [105], [106] | |||
| 3 | Merimepodib | Drug Info | [108] | |||
| 4 | Pertuzumab | Drug Info | [109], [110] | |||
| 5 | Tucatinib | Drug Info | [16] | |||
| 6 | HKI-272 | Drug Info | [113] | |||
| 7 | Nelipepimut S | Drug Info | [114] | |||
| 8 | Taxol/Paraplatin/Herceptin | Drug Info | [117] | |||
| 9 | AZD8931 | Drug Info | [122], [123] | |||
| 10 | BMS-599626 | Drug Info | [113] | |||
| 11 | CI-1033 | Drug Info | [113] | |||
| 12 | CP-724714 | Drug Info | [113] | |||
| 13 | HER-2 Protein AutoVac | Drug Info | [37] | |||
| 14 | Her2-targeted autologous T-cell therapy | Drug Info | [125] | |||
| 15 | HM-78136B | Drug Info | [127] | |||
| 16 | KN026 | Drug Info | [128] | |||
| 17 | Pazopanib + Tyverb/Tykerb | Drug Info | [108] | |||
| 18 | Tarloxotinib | Drug Info | [131] | |||
| 19 | AGN-208397 | Drug Info | [135] | |||
| 20 | AVX901 | Drug Info | [55] | |||
| 21 | BDTX-189 | Drug Info | [136] | |||
| 22 | ISB 1302 | Drug Info | [137] | |||
| 23 | Sym013 | Drug Info | [19] | |||
| 24 | TAS-0728 | Drug Info | [138] | |||
| 25 | Zenocutuzomab | Drug Info | [139] | |||
| 26 | S-222611 | Drug Info | [63] | |||
| 27 | BAY 2701439 | Drug Info | [143] | |||
| 28 | DZD1516 | Drug Info | [73] | |||
| 29 | GBR1302 | Drug Info | [144] | |||
| 30 | JNJ-26483327 | Drug Info | [146] | |||
| 31 | M802 | Drug Info | [147] | |||
| 32 | MBS301 | Drug Info | [149] | |||
| 33 | MT-5111 | Drug Info | [151] | |||
| 34 | TAK-285 | Drug Info | [156] | |||
| 35 | ZW49 | Drug Info | [160] | |||
| 36 | TAK165 | Drug Info | [113] | |||
| 37 | GW-974 | Drug Info | [162] | |||
| 38 | (1-Benzyl-1H-indazol-5-yl)-quinazolin-4-yl-amine | Drug Info | [163] | |||
| 39 | (1-Benzyl-1H-indol-5-yl)-quinazolin-4-yl-amine | Drug Info | [163] | |||
| Antagonist | [+] 4 Antagonist drugs | + | ||||
| 1 | Dacomitinib | Drug Info | [104] | |||
| 2 | NERATINIB MALEATE | Drug Info | [11] | |||
| 3 | PF-05280014 | Drug Info | [115] | |||
| 4 | MCLA-128 | Drug Info | [19] | |||
| Modulator | [+] 11 Modulator drugs | + | ||||
| 1 | Masoprocol | Drug Info | [107] | |||
| 2 | Varlitinib | Drug Info | [120] | |||
| 3 | DN24-02 | Drug Info | [124] | |||
| 4 | MGAH22 | Drug Info | [129] | |||
| 5 | MM-111 | Drug Info | [130] | |||
| 6 | ARRY-380 | Drug Info | [142] | |||
| 7 | Cipatinib | Drug Info | [120] | |||
| 8 | CUDC-101 | Drug Info | [72] | |||
| 9 | MEDI4276 | Drug Info | [19] | |||
| 10 | MM-302 | Drug Info | [150] | |||
| 11 | PF 5208766 | Drug Info | [164] | |||
| Enhancer | [+] 1 Enhancer drugs | + | ||||
| 1 | ABY-025 | Drug Info | [134] | |||
| CAR-T-Cell-Therapy | [+] 8 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | Anti-HER2 CAR-T | Drug Info | [52] | |||
| 2 | CART-HER-2 | Drug Info | [58] | |||
| 3 | HER-2-targeting CAR T Cells | Drug Info | [59] | |||
| 4 | CAR-T Cells targeting HER2 | Drug Info | [69] | |||
| 5 | CAR-T cells targeting HER2 | Drug Info | [70] | |||
| 6 | HER2-CAR T Cells | Drug Info | [76] | |||
| 7 | HER2-specific CAR T cell | Drug Info | [77] | |||
| 8 | HER2-specific T cells | Drug Info | [78] | |||
| Immunomodulator | [+] 1 Immunomodulator drugs | + | ||||
| 1 | AU105 | Drug Info | [53] | |||
| Binder | [+] 1 Binder drugs | + | ||||
| 1 | TA1-RTA | Drug Info | [166] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: TAK-285 | Ligand Info | |||||
| Structure Description | HER2 Kinase Domain Complexed with TAK-285 | PDB:3RCD | ||||
| Method | X-ray diffraction | Resolution | 3.21 Å | Mutation | No | [168] |
| PDB Sequence |
ALLRILKETE
719 LRKVKVLGSG729 AFGTVYKGIW739 IPDGENVKIP749 VAIKVLRPKA763 NKEILDEAYV 773 MAGVGSPYVS783 RLLGICLTST793 VQLVTQLMPY803 GCLLDHVREN813 RGRLGSQDLL 823 NWCMQIAKGM833 SYLEDVRLVH843 RDLAARNVLV853 KSPNHVKITD863 FGLVPIKWMA 890 LESILRRRFT900 HQSDVWSYGV910 TVWELMTFGA920 KPYDGIPARE930 IPDLLEKGER 940 LPQPPICTID950 VYMIMVKCWM960 IDSECRPRFR970 ELVSEFSRMA980 RDPQRFVVIQ 990 NLDSTFYRSL1008 LEDDDLVDAE1021 E
|
|||||
|
|
LEU726
3.861
GLY727
3.461
SER728
4.358
GLY729
4.041
VAL734
3.439
ALA751
3.418
ILE752
4.305
LYS753
3.419
MET774
4.518
SER783
2.899
ARG784
3.675
LEU785
3.064
LEU796
3.465
|
|||||
| Ligand Name: Beta-D-Mannose | Ligand Info | |||||
| Structure Description | HuA21-scFv in complex with the extracellular domain(ECD) of HER2 | PDB:6J71 | ||||
| Method | X-ray diffraction | Resolution | 2.92 Å | Mutation | No | [169] |
| PDB Sequence |
STQVCTGTDM
31 KLRLPASPET41 HLDMLRHLYQ51 GCQVVQGNLE61 LTYLPTNASL71 SFLQDIQEVQ 81 GYVLIAHNQV91 RQVPLQRLRI101 VRGTQLFEDN111 YALAVLDNGD121 PLNNTTPVTG 131 ASPGGLRELQ141 LRSLTEILKG151 GVLIQRNPQL161 CYQDTILWKD171 IFHKNNQLAL 181 TLIDTNRSRA191 CHPCSPMCKG201 SRCWGESSED211 CQSLTRTVCA221 GGCARCKGPL 231 PTDCCHEQCA241 AGCTGPKHSD251 CLACLHFNHS261 GICELHCPAL271 VTYNTDTFES 281 MPNPEGRYTF291 GASCVTACPY301 NYLSTDVGSC311 TLVCPLHNQE321 VTAEDGTQRC 331 EKCSKPCARV341 CYGLGMEHLR351 EVRAVTSANI361 QEFAGCKKIF371 GSLAFLPESF 381 DGDPASNTAP391 LQPEQLQVFE401 TLEEITGYLY411 ISAWPDSLPD421 LSVFQNLQVI 431 RGRILHNGAY441 SLTLQGLGIS451 WLGLRSLREL461 GSGLALIHHN471 THLCFVHTVP 481 WDQLFRNPHQ491 ALLHTANRPE501 DECVGEGLAC511 HQLCARGHCW521 GPGPTQCVNC 531 SQFLRGQECV541 EECRVLQGLP551 REYVNARHCL561 PCHPECQPQN571 GSVTCFGPEA 581 DQCVACAHYK591 DPPFCVARCP601 SGVKPDLSYM611 PIWKFPDEEG621 ACQPCPINCT 631 HSCVDLDD
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
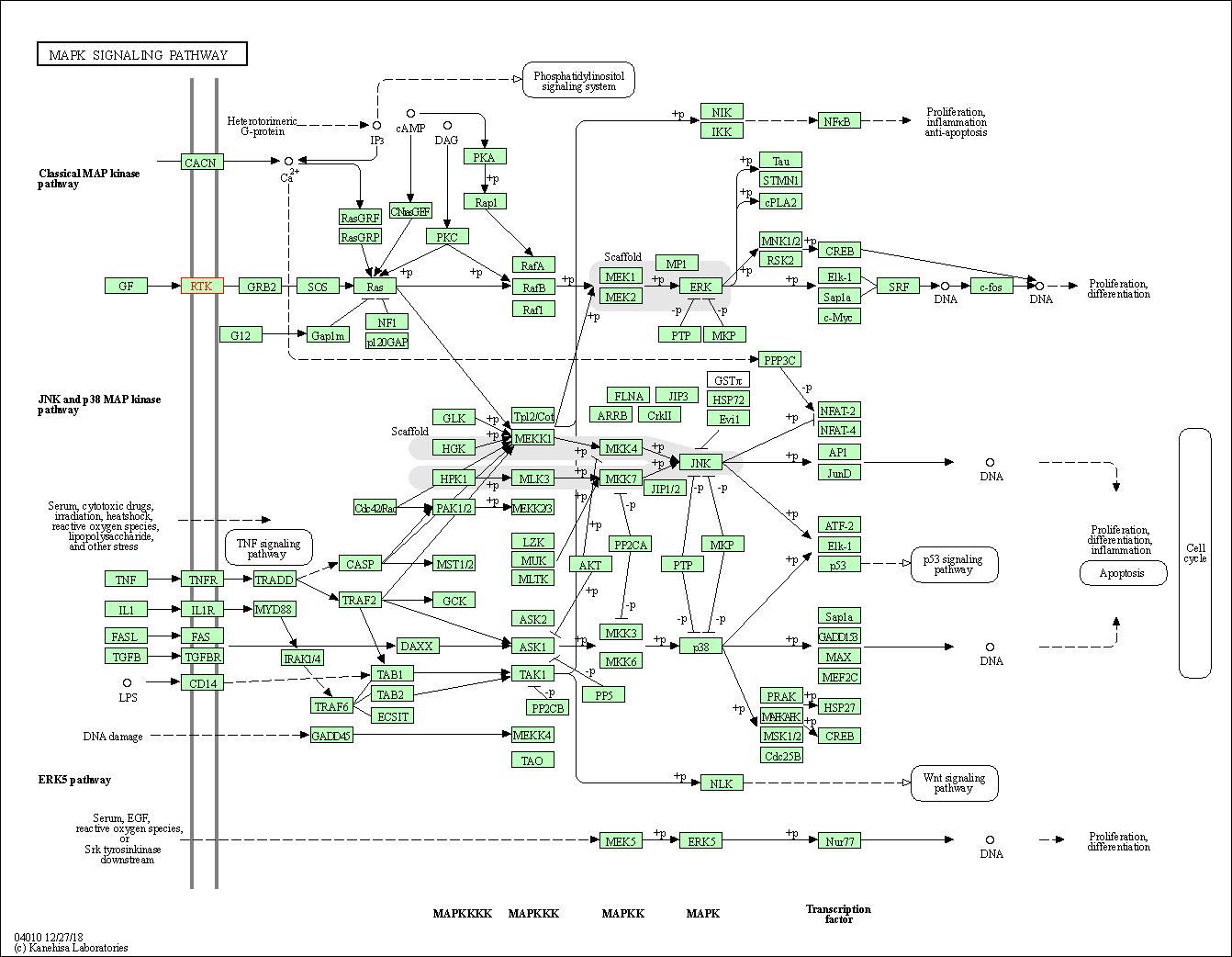
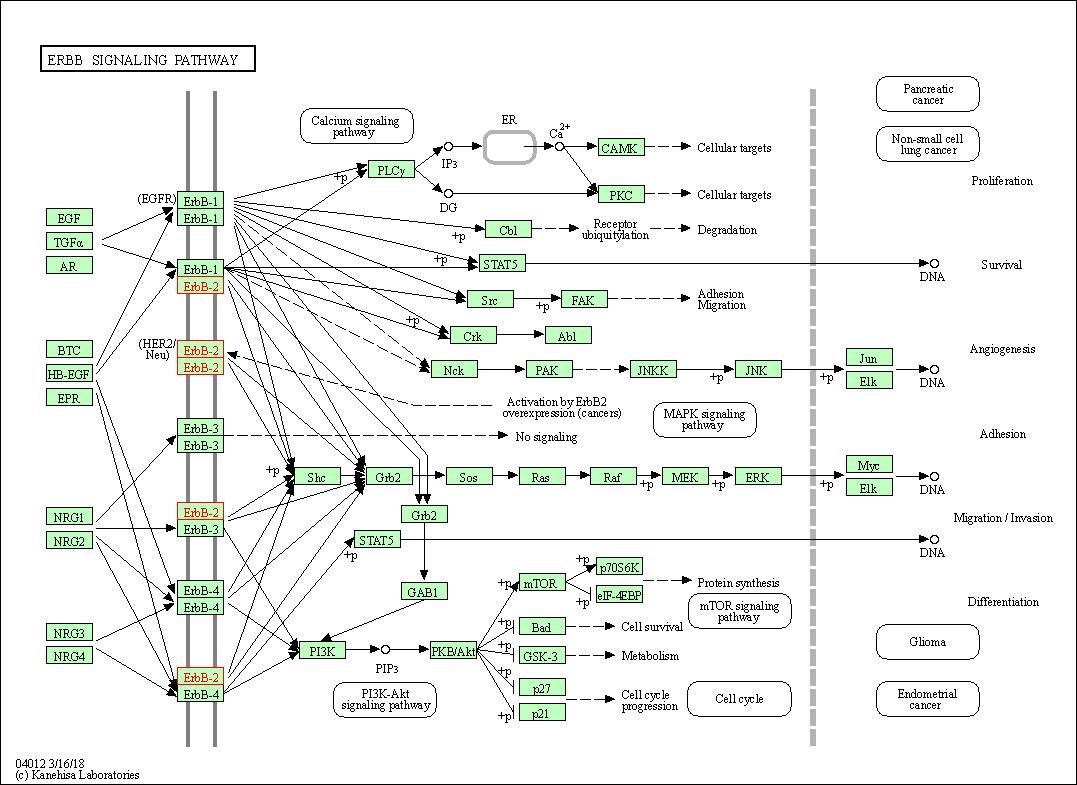
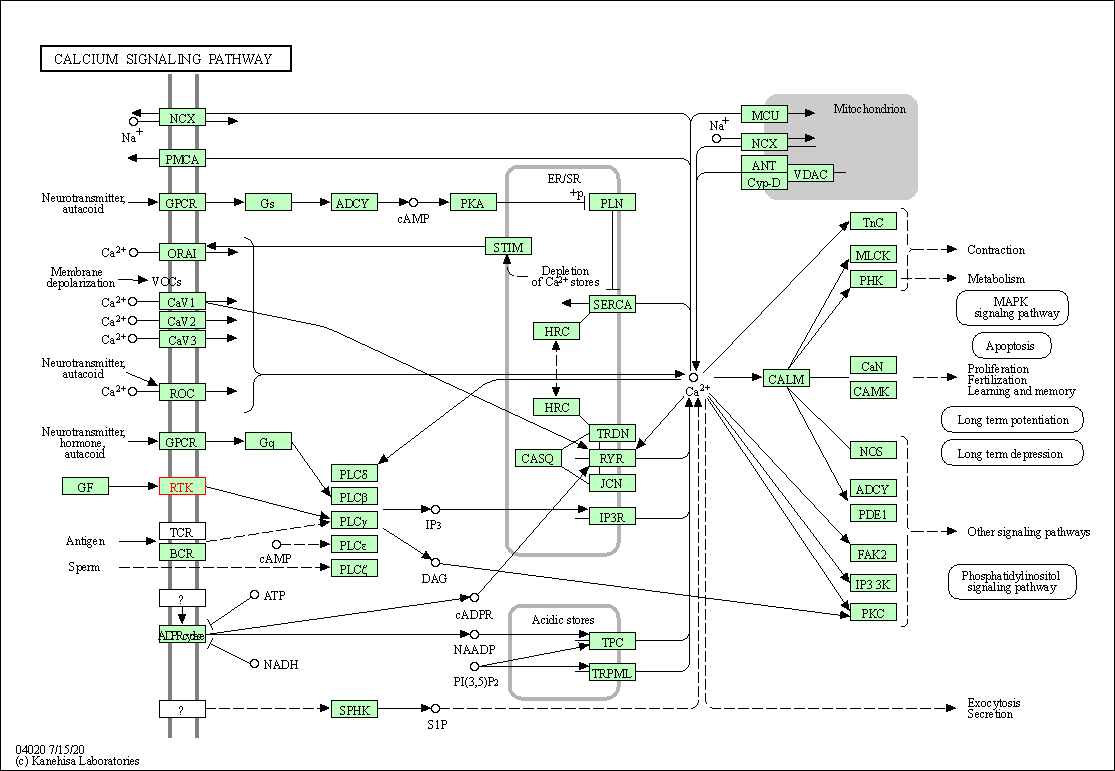
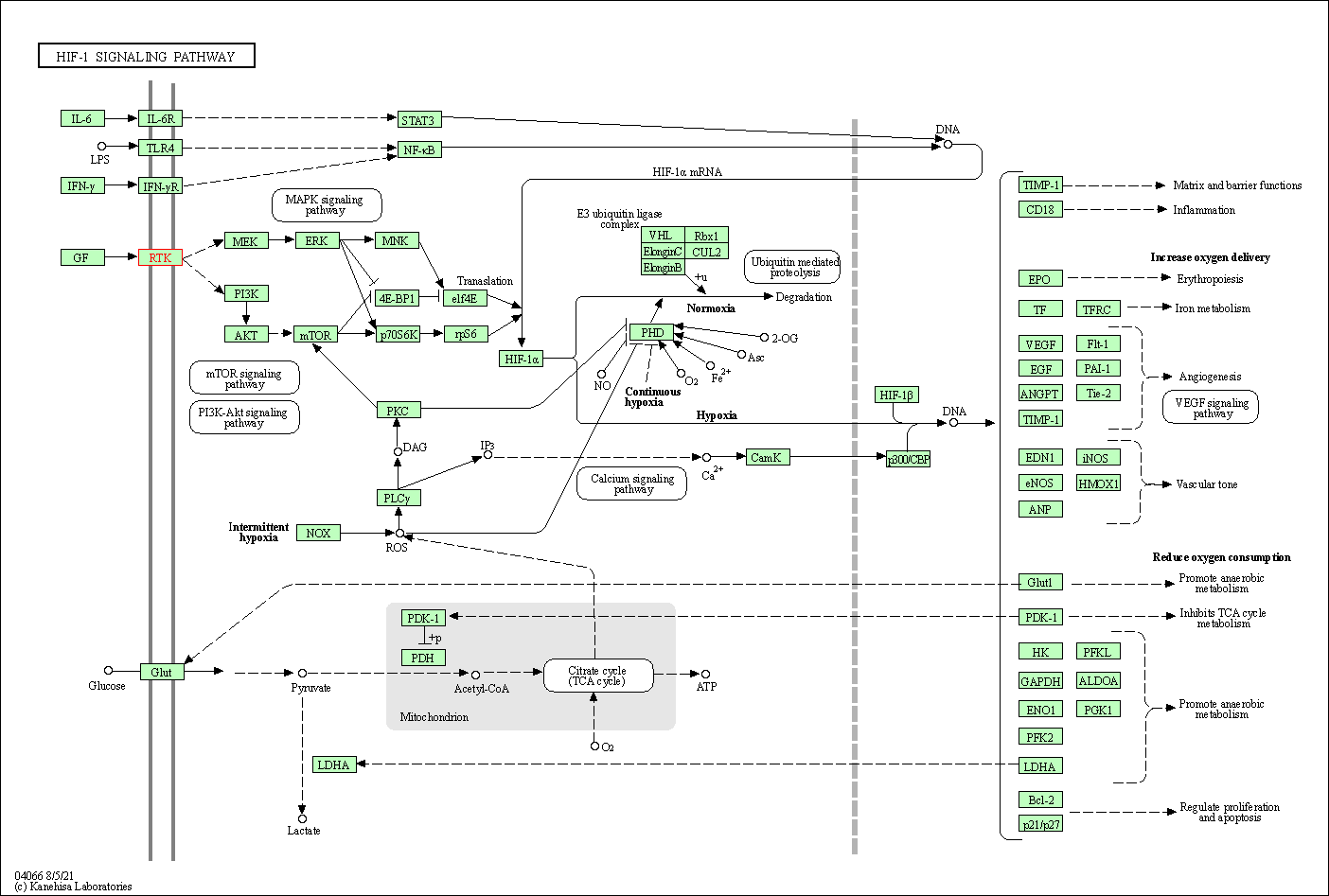
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| ErbB signaling pathway | hsa04012 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| HIF-1 signaling pathway | hsa04066 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
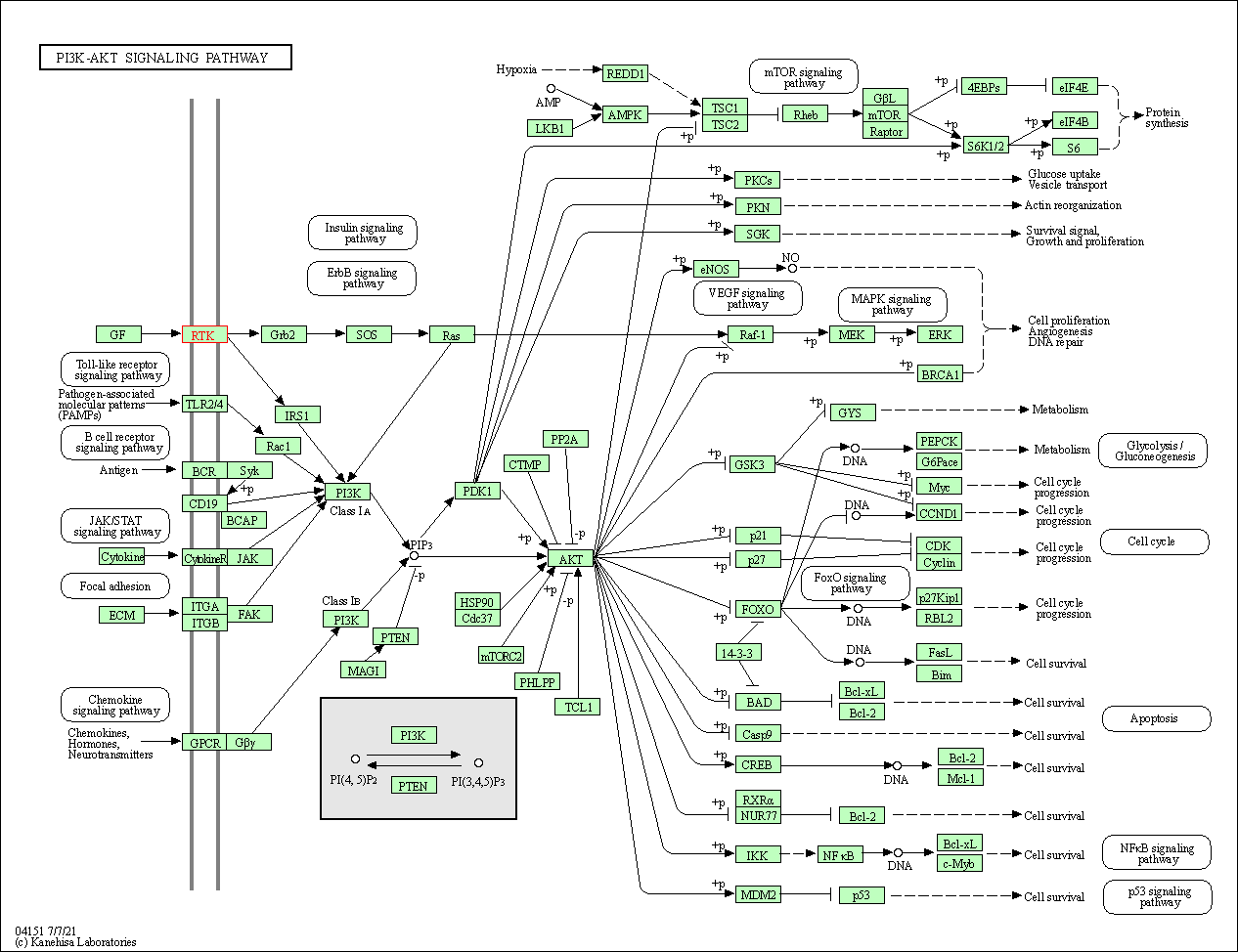
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
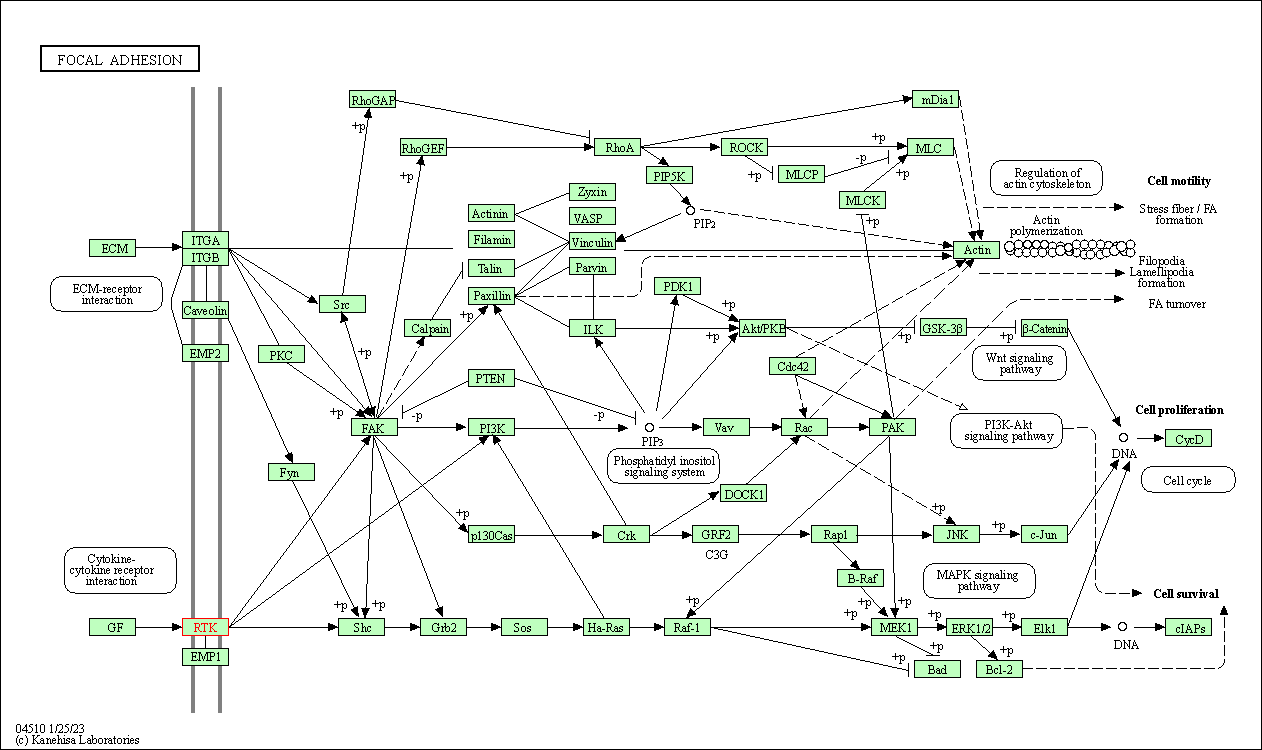
| Focal adhesion | hsa04510 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
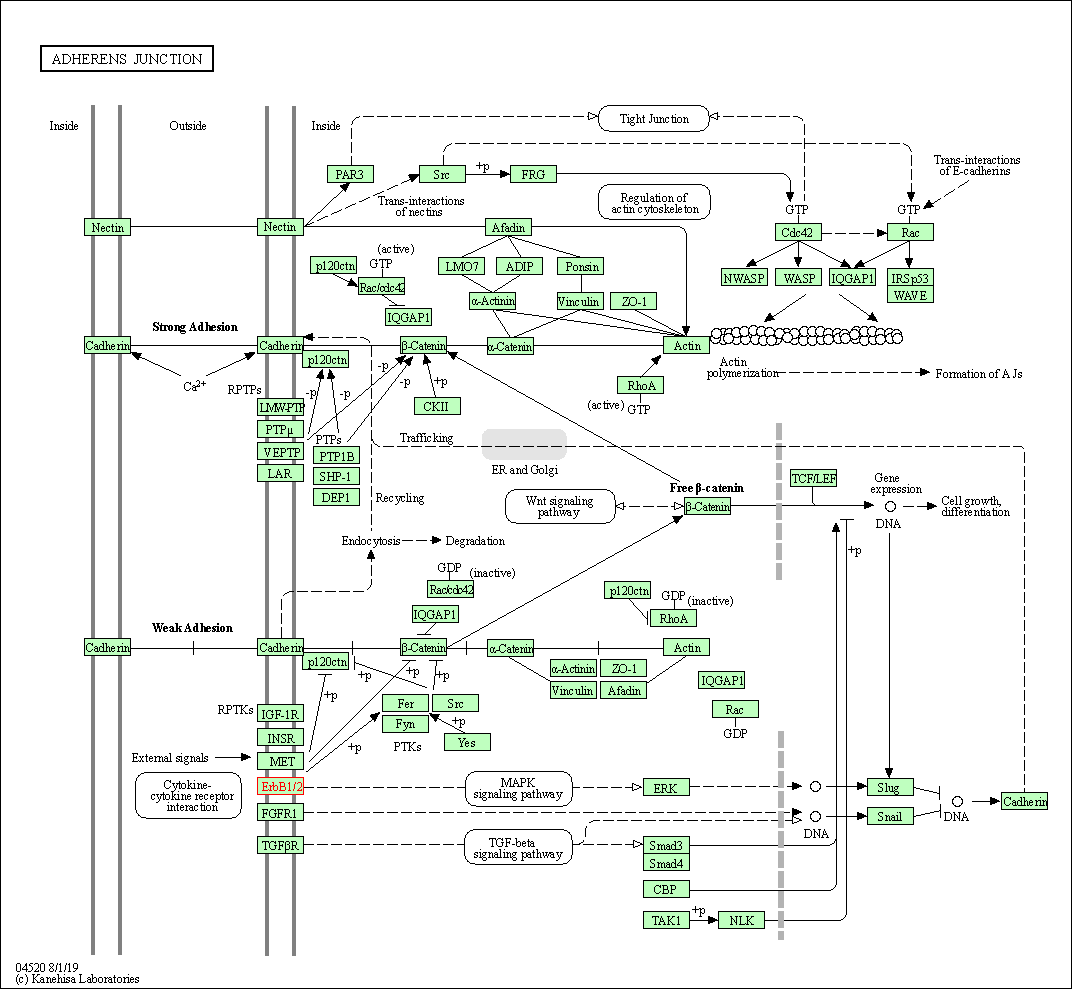
| Adherens junction | hsa04520 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
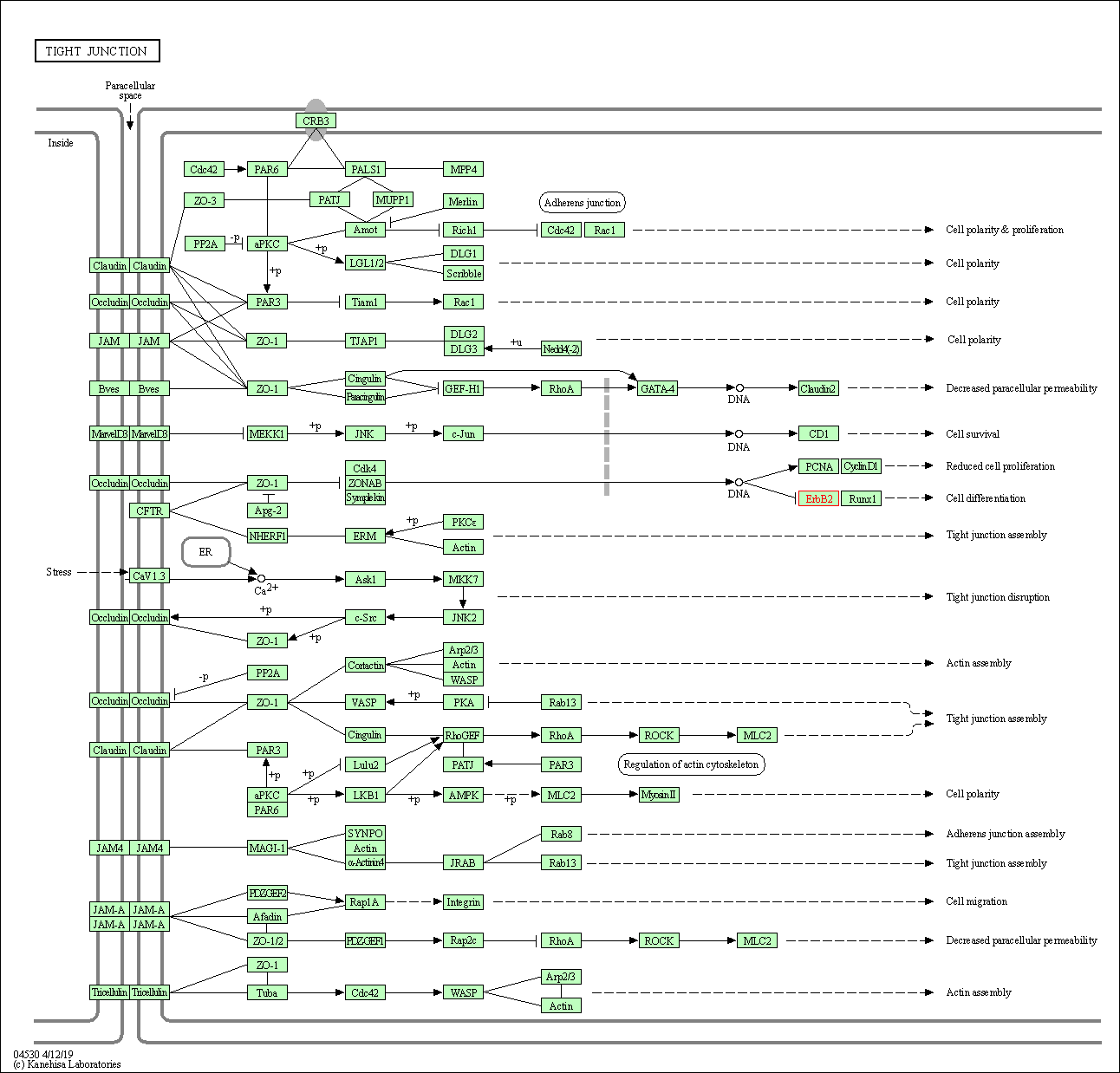
| Tight junction | hsa04530 | Affiliated Target |

|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 43 | Degree centrality | 4.62E-03 | Betweenness centrality | 1.76E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.60E-01 | Radiality | 1.45E+01 | Clustering coefficient | 2.25E-01 |
| Neighborhood connectivity | 5.21E+01 | Topological coefficient | 5.67E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Drug Resistance Mutation (DRM) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Her2/neu is not a commonly expressed therapeutic target in melanoma -- a large cohort tissue microarray study. Melanoma Res. 2004 Jun;14(3):207-10. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5667). | |||||
| REF 3 | BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors. Curr Opin Investig Drugs. 2008 Dec;9(12):1336-46. | |||||
| REF 4 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5692). | |||||
| REF 6 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 022059. | |||||
| REF 7 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4265). | |||||
| REF 9 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 10 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 022059. | |||||
| REF 11 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5046). | |||||
| REF 13 | Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013 May;14(6):461-71. | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5082). | |||||
| REF 15 | Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651. | |||||
| REF 16 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||||
| REF 17 | AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013 May 10;31(14):1719-25. | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021154) | |||||
| REF 19 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 20 | ClinicalTrials.gov (NCT01479244) Efficacy and Safety Study of NeuVax (Nelipepimut-S or E75) Vaccine to Prevent Breast Cancer Recurrence. U.S. National Institutes of Health. | |||||
| REF 21 | ClinicalTrials.gov (NCT02187744) A Study Of PF-05280014 Or Trastuzumab Plus Taxotere And Carboplatin In HER2 Positive Breast Cancer In The Neoadjuvant Setting (REFLECTIONS B327-04). U.S. National Institutes of Health. | |||||
| REF 22 | ClinicalTrials.gov (NCT03262935) A Multi-centre, Open-label, Randomized Clinical Trial Comparing the Efficacy and Safety of the Antibody-drug Conjugate SYD985 to Physician's Choice in Patients With HER2-positive Unresectable Locally Advanced or Metastatic Breast Cancer. U.S.National Institutes of Health. | |||||
| REF 23 | Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006 Jun 20;24(18):2786-92. | |||||
| REF 24 | Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer. 2014 Mar 1;120(5):642-51. | |||||
| REF 25 | Journal of Clinical Oncology, 2008 ASCO Annual Meeting Proceedings (Post-Meeting Edition), Vol 26, No 15S (May 20 Supplement), 2008: 3034. | |||||
| REF 26 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7717). | |||||
| REF 27 | ClinicalTrials.gov (NCT02117167) Intergroup Trial UNICANCER UC 0105-1305/ IFCT 1301: Efficacy of Targeted Drugs Guided by Genomic Profils in Metastatic NSCLC Patients. U.S. National Institutes of Health. | |||||
| REF 28 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7647). | |||||
| REF 29 | ClinicalTrials.gov (NCT01614522) A Clinical Trial Evaluating the Effect of ASLAN001 in Patients With Recurrent/Metastatic Gastric Cancer Whose Tumors Are Either HER-2 Amplified or Co-expressing HER-1and HER-2. U.S. National Institutes of Health. | |||||
| REF 30 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5675). | |||||
| REF 31 | Tyrosine kinase inhibitors. 17. Irreversible inhibitors of the epidermal growth factor receptor: 4-(phenylamino)quinazoline- and 4-(phenylamino)pyrido[3,2-d]pyrimidine-6-acrylamides bearing additional solubilizing functions. J Med Chem. 2000 Apr 6;43(7):1380-97. | |||||
| REF 32 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7883). | |||||
| REF 33 | ClinicalTrials.gov (NCT00102895) A Research Study of CP-724,714 in Patients With HER2 Overexpressing Metastatic Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 34 | ClinicalTrials.gov (NCT01353222) DN24-02 as Adjuvant Therapy in Subjects With High Risk HER2+ Urothelial Carcinoma. U.S. National Institutes of Health. | |||||
| REF 35 | Trifunctional antibody ertumaxomab: Non-immunological effects on Her2 receptor activity and downstream signaling. MAbs. 2012 Sep-Oct;4(5):614-22. | |||||
| REF 36 | Ertumaxomab: a trifunctional antibody for breast cancer treatment. Expert Opin Investig Drugs. 2008 Oct;17(10):1553-8. | |||||
| REF 37 | Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011 Nov 29;9(1):16-32. | |||||
| REF 38 | Adoptive cell therapies for glioblastoma. Front Oncol. 2013 Nov 11;3:275. | |||||
| REF 39 | ClinicalTrials.gov (NCT01729884) Vaccine Therapy in Treating Patients With Stage IV Hormone Receptor Positive Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 40 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7903). | |||||
| REF 41 | ClinicalTrials.gov (NCT02216916) Phase II Trial of HM781-36B in Patients With Metastatic/Recurrent Head and Neck Squamous Cell Carcinoma (HNSCC) After Failure of or Unfit for Platinum-containing Therapy. U.S. National Institutes of Health. | |||||
| REF 42 | ClinicalTrials.gov (NCT04881929) Study of KN026 in Combination With Docetaxel as Neoadjuvant Therapy in HER2-positive Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 43 | ClinicalTrials.gov (NCT01828021) Phase 2 Study of the Monoclonal Antibody MGAH22 (Margetuximab) in Patients With Relapsed or Refractory Advanced Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 44 | ClinicalTrials.gov (NCT01774851) A Study of MM-111 and Paclitaxel With Trastuzumab in Patients HER2 Positive Carcinomas of the Distal Esophagus, Gastroesophageal (GE) Junction and Stomach. U.S. National Institutes of Health. | |||||
| REF 45 | ClinicalTrials.gov (NCT04839510) A Study of MRG002 in the Treatment of HER2-positive Unresectable Locally Advanced or Metastatic Urothelium Cancer. U.S. National Institutes of Health. | |||||
| REF 46 | A randomized phase II study of lapatinib + pazopanib versus lapatinib in patients with HER2+ inflammatory breast cancer. Breast Cancer Res Treat. 2013 Jan;137(2):471-82. | |||||
| REF 47 | ClinicalTrials.gov (NCT02454842) Study for Treatment of Patients With EGFR Mutant, T790M-negative NSCLC (TH-4000). U.S. National Institutes of Health. | |||||
| REF 48 | ClinicalTrials.gov (NCT02912949) A Phase I/II Study of MCLA-128, a Full Length IgG1 Bispecific Antibody Targeting HER2 and HER3, in Patients With Solid Tumors (eNRGy). U.S.National Institutes of Health. | |||||
| REF 49 | ClinicalTrials.gov (NCT03602079) Study of A166 in Patients With Relapsed/Refractory Cancers Expressing HER2 Antigen or Having Amplified HER2 Gene. U.S. National Institutes of Health. | |||||
| REF 50 | ClinicalTrials.gov (NCT01858116) PET Study of Breast Cancer Patients Using [68Ga]ABY-025. U.S. National Institutes of Health. | |||||
| REF 51 | ClinicalTrials.gov (NCT01027650) Safety and Efficacy of AGN208397 in the Treatment of Macular Edema Associated With Retinal Vein Occlusion (RVO). U.S. National Institutes of Health. | |||||
| REF 52 | ClinicalTrials.gov (NCT02713984) A Clinical Research of CAR T Cells Targeting HER2 Positive Cancer | |||||
| REF 53 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 54 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 55 | ClinicalTrials.gov (NCT01526473) A Phase I Study To Evaluate The Antitumor Activity And Safety Of AVX901. U.S. National Institutes of Health. | |||||
| REF 56 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 57 | ClinicalTrials.gov (NCT04209465) A Study of BDTX-189, an Orally Available Allosteric ErbB Inhibitor, in Patients With Advanced Solid Tumors. (MasterKey-01). U.S. National Institutes of Health. | |||||
| REF 58 | ClinicalTrials.gov (NCT01935843) Treatment of Chemotherapy Refractory Human Epidermalgrowth Factor Receptor-2( HER-2) Positive Advanced Solid Tumors | |||||
| REF 59 | ClinicalTrials.gov (NCT02547961) Chimeric Antigen Receptor-Modified T Cells for Breast Cancer | |||||
| REF 60 | ClinicalTrials.gov (NCT03983395) Study of ISB 1302 (CD3 Bispecific Ab) in HER2-positive Metastatic Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 61 | ClinicalTrials.gov (NCT03410927) A Study of TAS0728 in Patients With Solid Tumors With HER2 or HER3 Abnormalities. U.S. National Institutes of Health. | |||||
| REF 62 | ClinicalTrials.gov (NCT03321981) MCLA-128 With Trastuzumab/Chemotherapy in HER2+ and With Endocrine Therapy in ER+ and Low HER2 Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 63 | Clinical pipeline report, company report or official report of Shionogi (2011). | |||||
| REF 64 | ClinicalTrials.gov (NCT04319757) A Phase I, Open Label, Dose Escalation Study of ACE1702 Cell Immunotherapy in Subjects With Advanced or Metastatic HER2-expressing Solid Tumors. U.S.National Institutes of Health. | |||||
| REF 65 | ClinicalTrials.gov (NCT04469127) A Study of AIP-303 in HER2 Positive Breast Cancer and/or Metastatic Breast Cancer Patients (Heroine01). U.S. National Institutes of Health. | |||||
| REF 66 | ClinicalTrials.gov (NCT01921335) ARRY-380 + Trastuzuamab for Breast w/ Brain Mets. U.S. National Institutes of Health. | |||||
| REF 67 | ClinicalTrials.gov (NCT04147819) A First in Human Study of BAY2701439 to Look at Safety, How the Body Absorbs, Distributes and Excretes the Drug, and How Well the Drug Works in Participants With Advanced Cancer Expressing the HER2 Protein. U.S. National Institutes of Health. | |||||
| REF 68 | ClinicalTrials.gov (NCT04257110) A First-in-human, Open Label, Multiple Dose, Dose Escalation and Cohort Expansion Phase I Study to Investigate the Safety, Tolerability, Pharmacokinetics and Antitumor Activities of BB-1701 in Subjects With Locally Advanced/Metastatic HER2 Expressing Solid Tumors. U.S.National Institutes of Health. | |||||
| REF 69 | ClinicalTrials.gov (NCT03267173) Evaluate the Safety and Efficacy of CAR-T in the Treatment of Pancreatic Cancer. | |||||
| REF 70 | ClinicalTrials.gov (NCT03198052) HER2/Mesothelin/Lewis-Y/PSCA/MUC1/PD-L1/CD80/86-CAR-T Cells Immunotherapy Against Cancers | |||||
| REF 71 | ClinicalTrials.gov (NCT01301911) Study of Cipatinib in Patients With HER2 Positive or Uncertain Advanced Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 72 | A Phase I Study of CUDC-101, a Multitarget Inhibitor of HDACs, EGFR, and HER2, in Combination with Chemoradiation in Patients with Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2015 Apr 1;21(7):1566-73. | |||||
| REF 73 | ClinicalTrials.gov (NCT04509596) DZD1516 in Combination With Trastuzumab and Capecitabine, or in Combination With T-DM1, in Patients With Metastatic HER2 Positive Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 74 | ClinicalTrials.gov (NCT02829372) Phase 1 Study of Single Agent GBR 1302 in Subjects With HER2 Positive Cancers (GBR 1302-101). U.S. National Institutes of Health. | |||||
| REF 75 | ClinicalTrials.gov (NCT04450732) Safety of GQ1001 in Adult Patients With HER2-Positive Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 76 | ClinicalTrials.gov (NCT03696030) HER2-CAR T Cells in Treating Participants With Brain or Leptomeningeal Metastases | |||||
| REF 77 | ClinicalTrials.gov (NCT03500991) HER2-specific CAR T Cell Locoregional Immunotherapy for HER2-positive Recurrent/Refractory Pediatric CNS Tumors | |||||
| REF 78 | ClinicalTrials.gov (NCT02442297) T Cells Expressing HER2-specific Chimeric Antigen Receptors(CAR) for Patients With Glioblastoma | |||||
| REF 79 | Development of a cancer vaccine: peptides, proteins, and DNA. Cancer Chemother Pharmacol. 2000;46 Suppl:S77-82. | |||||
| REF 80 | ClinicalTrials.gov (NCT00676299) A Safety and Dose-finding Study of JNJ-26483327, a Drug in Development for Cancer, for Patients With Advanced and/or Refractory Solid Malignancies.. U.S. National Institutes of Health. | |||||
| REF 81 | ClinicalTrials.gov (NCT04501770) A Study of M802 (HER2 and CD3) in HER2-Positive Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 82 | ClinicalTrials.gov (NCT03696030) HER2-CAR T Cells in Treating Patients With Recurrent Brain or Leptomeningeal Metastases. U.S. National Institutes of Health. | |||||
| REF 83 | ClinicalTrials.gov (NCT03842085) Phase I Clinical Study of MBS301 in Treatment of HER2 Positive Recurrent or Metastatic Malignant Solid Tumor. U.S. National Institutes of Health. | |||||
| REF 84 | ClinicalTrials.gov (NCT01304797) Safety and Pharmacokinetic Study of MM-302 in Patients With Advanced Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 85 | ClinicalTrials.gov (NCT04029922) Study of MT-5111 in HER2-positive Solid Tumors (MT-5111). U.S. National Institutes of Health. | |||||
| REF 86 | ClinicalTrials.gov (NCT01152398) A Safety and Immunology Study of a Modified Vaccinia Vaccine for HER-2(+) Breast Cancer After Adjuvant Therapy. U.S. National Institutes of Health. | |||||
| REF 87 | ClinicalTrials.gov (NCT03696771) Study to Determine Safety and Dose of NJH395 in Non-breast HER2+ Advanced Cancer. U.S. National Institutes of Health. | |||||
| REF 88 | Clinical pipeline report, company report or official report of Zensun. | |||||
| REF 89 | ClinicalTrials.gov (NCT03448042) A Phase I Study of BTRC4017A in Participants With Locally Advanced or Metastatic HER2-Expressing Cancers. U.S. National Institutes of Health. | |||||
| REF 90 | Clinical pipeline report, company report or official report of Sanofi | |||||
| REF 91 | ClinicalTrials.gov (NCT04460456) A Study of SBT6050 Alone and in Combination With Pembrolizumab in Patients With Advanced HER2 Expressing Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 92 | ClinicalTrials.gov (NCT00535522) A Safety and Pharmacokinetic Study of TAK-285 in Patients With Advanced Cancer. U.S. National Institutes of Health. | |||||
| REF 93 | ClinicalTrials.gov (NCT01409343) TrasGEX Dose Escalation Study. U.S. National Institutes of Health. | |||||
| REF 94 | Clinical pipeline report, company report or official report of Tessa Therapeutics | |||||
| REF 95 | ClinicalTrials.gov (NCT01895491) Safety Study of VM206RY in Subjects With Expression of HER2 in Breast Cancer. U.S. National Institutes of Health. | |||||
| REF 96 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026315) | |||||
| REF 97 | ClinicalTrials.gov (NCT03821233) A Dose Finding Study of ZW49 in Patients With HER2-Positive Cancers. U.S. National Institutes of Health. | |||||
| REF 98 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028450) | |||||
| REF 99 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6011). | |||||
| REF 100 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019257) | |||||
| REF 101 | Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020 Sep;19(9):589-608. | |||||
| REF 102 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010108) | |||||
| REF 103 | Boehringer Ingelheim. Product Development Pipeline. June 2 2009. | |||||
| REF 104 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7422). | |||||
| REF 105 | Triple negative breast cancer--current status and prospective targeted treatment based on HER1 (EGFR), TOP2A and C-MYC gene assessment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009 Mar;153(1):13-7. | |||||
| REF 106 | Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007 Jan;12(1-2):34-42. | |||||
| REF 107 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 108 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 109 | Clinical pipeline report, company report or official report of Roche (2009). | |||||
| REF 110 | Pertuzumab. Drugs Fut 2008, 33(2): 123 ISSN 0377-8282. | |||||
| REF 111 | Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | |||||
| REF 112 | Anti-ErbB-2 monoclonal antibodies and ErbB-2-directed vaccines. Cancer Immunol Immunother. 2002 Jan;50(11):569-87. | |||||
| REF 113 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||||
| REF 114 | The HER2 peptide nelipepimut-S (E75) vaccine (NeuVax in breast cancer patients at risk for recurrence: correlation of immunologic data with clinical response. Immunotherapy. 2014;6(5):519-31. | |||||
| REF 115 | Comparative nonclinical assessments of the proposed biosimilar PF-05280014 and trastuzumab (Herceptin( )). BioDrugs. 2014 Oct;28(5):451-9. | |||||
| REF 116 | Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur J Med Chem. 2019 Dec 1;183:111682. | |||||
| REF 117 | Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer. 2005 Dec;6(5):425-32. | |||||
| REF 118 | Clinical pipeline report, company report or official report of Genentech (2009). | |||||
| REF 119 | Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007 Jul 5;357(1):39-51. | |||||
| REF 120 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 121 | Use of anti-CD3 x anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu+ tumors. J Hematother Stem Cell Res. 2001 Apr;10(2):247-60. | |||||
| REF 122 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||||
| REF 123 | Characterization of AZD8931, a potent reversible small molecule inhibitor against epidermal growth factor receptor (EGFR), erythroblastic leukemia viral oncogene homolog 2 (HER2) and 3 (HER3) with a unique and balanced pharmacological profile. J Clin Oncol 27:15s, 2009. | |||||
| REF 124 | Treatment with autologous antigen-presenting cells activated with the HER-2 based antigen Lapuleucel-T: results of a phase I study in immunologic and clinical activity in HER-2 overexpressing breast cancer. J Clin Oncol. 2007 Aug 20;25(24):3680-7. | |||||
| REF 125 | A new hope in immunotherapy for malignant gliomas: adoptive T cell transfer therapy. J Immunol Res. 2014;2014:326545. | |||||
| REF 126 | N-terminally LRMK-linked HER-2 peptides, AE-37 [p776(774-788)] and AE-47 [Ava-F7(776-788)], aid differentiation of E75-TCR+CD8+ cells to perforin-positive cells. Anticancer Res. 2009 Jul;29(7):2427-35. | |||||
| REF 127 | Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int J Cancer. 2012 May 15;130(10):2445-54. | |||||
| REF 128 | Clinical pipeline report, company report or official report of Alphamab Oncology. | |||||
| REF 129 | Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011; 13(6): R123. | |||||
| REF 130 | Bispecific antibodies rise again. Nat Rev Drug Discov. 2014 Nov;13(11):799-801. | |||||
| REF 131 | Tarloxotinib Is a Hypoxia-Activated Pan-HER Kinase Inhibitor Active Against a Broad Range of HER-Family Oncogenes. Clin Cancer Res. 2021 Mar 1;27(5):1463-1475. | |||||
| REF 132 | Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov. 2022 May 2;12(5):1233-1247. | |||||
| REF 133 | Clinical pipeline report, company report or official report of KLUS Pharma. | |||||
| REF 134 | Targeting of HER2-expressing tumors using 111In-ABY-025, a second-generation affibody molecule with a fundamentally reengineered scaffold. J Nucl Med. 2010 Jul;51(7):1131-8. | |||||
| REF 135 | New hope for dry AMD. Nat Rev Drug Discov. 2013 Jul;12(7):501-2. | |||||
| REF 136 | Clinical pipeline report, company report or official report of Black Diamond Therapeutics. | |||||
| REF 137 | Clinical pipeline report, company report or official report of Ichnos Sciences. | |||||
| REF 138 | TAS0728, A Covalent-binding, HER2-selective Kinase Inhibitor Shows Potent Antitumor Activity in Preclinical Models. Mol Cancer Ther. 2019 Apr;18(4):733-742. | |||||
| REF 139 | Clinical pipeline report, company report or official report of Merus. | |||||
| REF 140 | A Novel off-the-Shelf Trastuzumab-Armed NK Cell Therapy (ACE1702) Using Antibody-Cell-Conjugation Technology. Cancers (Basel). 2021 May 31;13(11):2724. | |||||
| REF 141 | Clinical pipeline report, company report or official report of Adagene. | |||||
| REF 142 | ARRY-380, a Potent, Small Molecule Inhibitor of ErbB2, Increases Survival in Intracranial ErbB2+ Xenograft Models in Mice | |||||
| REF 143 | National Cancer Institute Drug Dictionary (drug name BAY2701439). | |||||
| REF 144 | Clinical pipeline report, company report or official report of Glenmark Pharmaceuticals. | |||||
| REF 145 | Clinical pipeline report, company report or official report of GeneQuantum Healthcare. | |||||
| REF 146 | National Cancer Institute Drug Dictionary (drug id 596693). | |||||
| REF 147 | A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells. J Exp Clin Cancer Res. 2019 Aug 14;38(1):355. | |||||
| REF 148 | Clinical pipeline report, company report or official report of Mustang Bio. | |||||
| REF 149 | Clinical pipeline report, company report or official report of Beijing Mabworks Biotech. | |||||
| REF 150 | Whole-body organ-level and kidney micro-dosimetric evaluations of (64)Cu-loaded HER2/ErbB2-targeted liposomal doxorubicin ((64)Cu-MM-302) in rodents and primates. EJNMMI Res. 2015 Apr 14;5:24. | |||||
| REF 151 | Clinical pipeline report, company report or official report of Molecular Templates. | |||||
| REF 152 | Active immunotherapy in HER2 overexpressing breast cancer: current status and future perspectives. Ann Oncol. 2013 Jul;24(7):1740-8. | |||||
| REF 153 | Clinical pipeline report, company report or official report of Zensun. | |||||
| REF 154 | Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023 Feb;22(2):101-126. | |||||
| REF 155 | Clinical pipeline report, company report or official report of Silverback Therapeutics. | |||||
| REF 156 | Clinical pipeline report, company report or official report of Takeda (2009). | |||||
| REF 157 | J Clin Oncol 32:5s, 2014 (suppl; abstr 2515). | |||||
| REF 158 | WO patent application no. 2014,1441,21, Classification and actionability indices for lung cancer. | |||||
| REF 159 | Immunotoxins and Anticancer Drug Conjugate Assemblies: The Role of the Linkage between Components. Toxins (Basel) 2011 July; 3(7): 848-883. | |||||
| REF 160 | Clinical pipeline report, company report or official report of Zymeworks. | |||||
| REF 161 | Cancer therapy with bispecific antibodies: Clinical experience. Curr Opin Mol Ther. 2010 June; 12(3): 340-349. | |||||
| REF 162 | WO patent application no. 2005,0443,02, Selective erbb2 inhibitor/anti-erbb antibody combinations in the treatment of cancer. | |||||
| REF 163 | Indazolylamino quinazolines and pyridopyrimidines as inhibitors of the EGFr and C-erbB-2. Bioorg Med Chem Lett. 2001 Jun 4;11(11):1401-5. | |||||
| REF 164 | Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor... J Med Chem. 2005 Feb 24;48(4):1107-31. | |||||
| REF 165 | A new human antitumor immunoreagent specific for ErbB2. Clin Cancer Res. 2002 Jun;8(6):1710-9. | |||||
| REF 166 | Synergistic interaction between anti-p185HER-2 ricin A chain immunotoxins and radionuclide conjugates for inhibiting growth of ovarian and breast cancer cells that overexpress HER-2. Clin Cancer Res.2000 Aug;6(8):3334-41. | |||||
| REF 167 | Clinical pipeline report, company report or official report of Biocytogen | |||||
| REF 168 | Design and synthesis of novel human epidermal growth factor receptor 2 (HER2)/epidermal growth factor receptor (EGFR) dual inhibitors bearing a pyrrolo[3,2-d]pyrimidine scaffold. J Med Chem. 2011 Dec 8;54(23):8030-50. | |||||
| REF 169 | Structural insight into a matured humanized monoclonal antibody HuA21 against HER2-overexpressing cancer cells. Acta Crystallogr D Struct Biol. 2019 Jun 1;75(Pt 6):554-563. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

