Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0C2NK
|
|||
| Former ID |
DIB020562
|
|||
| Drug Name |
norfluoxetine
|
|||
| Synonyms |
Norfluoxetine; Desmethylfluoxetine; Norfluoxetin; 56161-73-0; 83891-03-6; 3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine; WIQRCHMSJFFONW-UHFFFAOYSA-N; gamma-(4-(Trifluoromethyl)phenoxy)benzenepropanamine; norfluoxetine hcl; 3-Phenyl-3-(4-trifluoromethyl-phenoxy)-propylamine; 3-phenyl-3-(4-(trifluoromethyl)phenoxy)propan-1-amine; Benzenepropanamine, gamma-(4-(trifluoromethyl)phenoxy)-; benzenepropanamine, gamma-[4-(trifluoromethyl)phenoxy]-; ( inverted exclamation markA)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
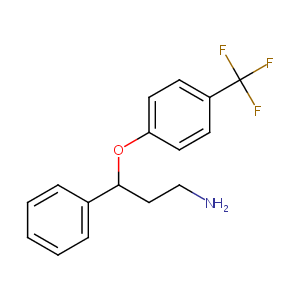 |
Download2D MOL
|
||
| Formula |
C16H16F3NO
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)C(CCN)OC2=CC=C(C=C2)C(F)(F)F
|
|||
| InChI |
1S/C16H16F3NO/c17-16(18,19)13-6-8-14(9-7-13)21-15(10-11-20)12-4-2-1-3-5-12/h1-9,15H,10-11,20H2
|
|||
| InChIKey |
WIQRCHMSJFFONW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 83891-03-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
515992, 841245, 3249471, 4712768, 8152798, 14849332, 29223632, 50066100, 50707944, 57322322, 85209721, 92729852, 99443277, 103131848, 103556000, 104306700, 117505046, 125607181, 125812945, 126665903, 129587205, 135062212, 135371955, 135650734, 137016413, 141566791, 152050510, 160967894, 162919696, 163640919, 179293685, 184565588, 184605154, 223682774, 223809731, 225228000, 226980400, 249897421, 252236770, 252355234
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 2A receptor (HTR2A) | Target Info | Antagonist | [2] |
| 5-HT 2B receptor (HTR2B) | Target Info | Antagonist | [2] | |
| 5-HT 2C receptor (HTR2C) | Target Info | Antagonist | [2] | |
| Voltage-gated potassium channel Kv3.1 (KCNC1) | Target Info | Inhibitor (gating inhibitor) | [3] | |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Gap junction | ||||
| Serotonergic synapse | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | 5HT2 type receptor mediated signaling pathway | |||
| Reactome | Serotonin receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Serotonin Receptor 2 and ELK-SRF/GATA4 signaling | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| Serotonin Receptor 2 and STAT3 Signaling | ||||
| SIDS Susceptibility Pathways | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 208). | |||
| REF 2 | Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000 Dec 5;102(23):2836-41. | |||
| REF 3 | Effects of norfluoxetine, the major metabolite of fluoxetine, on the cloned neuronal potassium channel Kv3.1. Neuropharmacology. 2001 Sep;41(4):443-53. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

