Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0D3AI
|
|||
| Former ID |
DCL000150
|
|||
| Drug Name |
L-NAME
|
|||
| Synonyms |
N-Nitroarginine methyl ester; NG-NITROARGININE METHYL ESTER; N(G)-Nitroarginine methyl ester; N-Nitro-L-arginine methylester; Ngamma-Nitro-L-arginine methyl ester; N(G)-Nitro-L-arginine methyl ester; N(sup G)-Nitro-L-arginine methyl ester; N-omega-Nitro-O-arginine methyl ester; Methyl (2S)-2-amino-5-[[amino(nitramido)methylidene]amino]pentanoate; Methyl N5-(N'-nitrocarbamimidoyl)-L-ornithinate; N(5)-(Imino(nitroamino)methyl)-L-ornithine methyl ester; N-.omega.-Nitro-O-arginine methyl ester; L-Ornithine, N(5)-(imino(nitroamino)methyl)-, methyl ester; Methyl (2S)-2-amino-5-[(amino-nitramido-methylidene)amino]pentanoate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertension [ICD-11: BA00-BA04] | Phase 2 | [1], [2] | |
| Company |
Registry Operator
|
|||
| Structure |
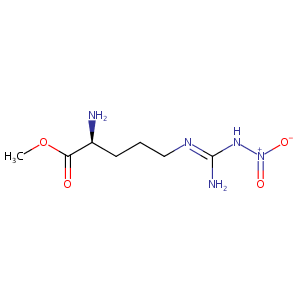 |
Download2D MOL |
||
| Formula |
C7H15N5O4
|
|||
| Canonical SMILES |
COC(=O)C(CCCN=C(N)N[N+](=O)[O-])N
|
|||
| InChI |
1S/C7H15N5O4/c1-16-6(13)5(8)3-2-4-10-7(9)11-12(14)15/h5H,2-4,8H2,1H3,(H3,9,10,11)/t5-/m0/s1
|
|||
| InChIKey |
KCWZGJVSDFYRIX-YFKPBYRVSA-N
|
|||
| CAS Number |
CAS 50903-99-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
6988, 799994, 11113631, 11120322, 11120810, 11121298, 11121786, 11122266, 11362975, 11365537, 11368099, 11371101, 11371102, 11373700, 11376261, 14717146, 14749398, 15121709, 15121710, 26751830, 26751831, 34705694, 47959523, 48034876, 48259010, 48259011, 49737752, 50104531, 50104532, 50104533, 56310758, 56311189, 56311968, 57312345, 59831004, 76083057, 87550947, 90340714, 92310137, 99301049, 99302569, 103166542, 103995882, 104332835, 119503169, 124750046, 128364160, 134338830, 135001289, 135654724
|
|||
| ChEBI ID |
CHEBI:7549
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5213). | |||
| REF 2 | Inhibition of nitric oxide synthase by L-NAME speeds phase II pulmonary .VO2 kinetics in the transition to moderate-intensity exercise in man. J Physiol. 2003 Oct 1;552(Pt 1):265-72. | |||
| REF 3 | Counter-regulation by atorvastatin of gene modulations induced by L-NAME hypertension is associated with vascular protection. Vascul Pharmacol. 2009 Oct;51(4):253-61. | |||
| REF 4 | Enhanced pulmonary expression of the TrkB neurotrophin receptor in hypoxic rats is associated with increased acetylcholine-induced airway contracti... Acta Physiol (Oxf). 2009 Nov;197(3):253-64. | |||
| REF 5 | L-NAME causes antinociception by stimulation of the arginine-NO-cGMP pathway. Mediators Inflamm. 2000;9(1):25-30. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

