Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T06046
(Former ID: TTDC00097)
|
|||||
| Target Name |
Nitric-oxide synthase endothelial (NOS3)
|
|||||
| Synonyms |
Nitric oxide synthase, endothelial; NOSIII; NOS,type III; NOS type III; Endothelial nitric oxide synthase; Endothelial NOS; ENOS; EC-NOS; Constitutive NOS; CNOS
Click to Show/Hide
|
|||||
| Gene Name |
NOS3
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Myocardial infarction [ICD-11: BA41-BA43] | |||||
| Function |
NO mediates vascular endothelial growth factor (VEGF)-induced angiogenesis in coronary vessels and promotes blood clotting through the activation of platelets. Produces nitric oxide (NO) which is implicated in vascular smooth muscle relaxation through a cGMP-mediated signal transduction pathway.
Click to Show/Hide
|
|||||
| BioChemical Class |
Paired donor oxygen oxidoreductase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.14.13.39
|
|||||
| Sequence |
MGNLKSVAQEPGPPCGLGLGLGLGLCGKQGPATPAPEPSRAPASLLPPAPEHSPPSSPLT
QPPEGPKFPRVKNWEVGSITYDTLSAQAQQDGPCTPRRCLGSLVFPRKLQGRPSPGPPAP EQLLSQARDFINQYYSSIKRSGSQAHEQRLQEVEAEVAATGTYQLRESELVFGAKQAWRN APRCVGRIQWGKLQVFDARDCRSAQEMFTYICNHIKYATNRGNLRSAITVFPQRCPGRGD FRIWNSQLVRYAGYRQQDGSVRGDPANVEITELCIQHGWTPGNGRFDVLPLLLQAPDDPP ELFLLPPELVLEVPLEHPTLEWFAALGLRWYALPAVSNMLLEIGGLEFPAAPFSGWYMST EIGTRNLCDPHRYNILEDVAVCMDLDTRTTSSLWKDKAAVEINVAVLHSYQLAKVTIVDH HAATASFMKHLENEQKARGGCPADWAWIVPPISGSLTPVFHQEMVNYFLSPAFRYQPDPW KGSAAKGTGITRKKTFKEVANAVKISASLMGTVMAKRVKATILYGSETGRAQSYAQQLGR LFRKAFDPRVLCMDEYDVVSLEHETLVLVVTSTFGNGDPPENGESFAAALMEMSGPYNSS PRPEQHKSYKIRFNSISCSDPLVSSWRRKRKESSNTDSAGALGTLRFCVFGLGSRAYPHF CAFARAVDTRLEELGGERLLQLGQGDELCGQEEAFRGWAQAAFQAACETFCVGEDAKAAA RDIFSPKRSWKRQRYRLSAQAEGLQLLPGLIHVHRRKMFQATIRSVENLQSSKSTRATIL VRLDTGGQEGLQYQPGDHIGVCPPNRPGLVEALLSRVEDPPAPTEPVAVEQLEKGSPGGP PPGWVRDPRLPPCTLRQALTFFLDITSPPSPQLLRLLSTLAEEPREQQELEALSQDPRRY EEWKWFRCPTLLEVLEQFPSVALPAPLLLTQLPLLQPRYYSVSSAPSTHPGEIHLTVAVL AYRTQDGLGPLHYGVCSTWLSQLKPGDPVPCFIRGAPSFRLPPDPSLPCILVGPGTGIAP FRGFWQERLHDIESKGLQPTPMTLVFGCRCSQLDHLYRDEVQNAQQRGVFGRVLTAFSRE PDNPKTYVQDILRTELAAEVHRVLCLERGHMFVCGDVTMATNVLQTVQRILATEGDMELD EAGDVIGVLRDQQRYHEDIFGLTLRTQEVTSRIRTQSFSLQERQLRGAVPWAFDPPGSDT NSP Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A02149 ; BADD_A02564 ; BADD_A02600 | |||||
| HIT2.0 ID | T64UH1 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 6 Clinical Trial Drugs | + | ||||
| 1 | Tilarginine acetate | Drug Info | Phase 3 | Myocardial infarction | [2] | |
| 2 | ACCLAIM | Drug Info | Phase 2 | Coronary artery disease | [3] | |
| 3 | L-NAME | Drug Info | Phase 2 | Hypertension | [4], [5] | |
| 4 | MTR105 | Drug Info | Phase 2 | Hypotension | [6] | |
| 5 | VAS-203 | Drug Info | Phase 2 | Brain injury | [7] | |
| 6 | Autologous cell based gene therapy | Drug Info | Phase 1 | Pulmonary hypertension | [8] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Modulator | [+] 2 Modulator drugs | + | ||||
| 1 | Tilarginine acetate | Drug Info | [1] | |||
| 2 | Autologous cell based gene therapy | Drug Info | [15] | |||
| Stimulator | [+] 1 Stimulator drugs | + | ||||
| 1 | ACCLAIM | Drug Info | [9] | |||
| Inhibitor | [+] 85 Inhibitor drugs | + | ||||
| 1 | L-NAME | Drug Info | [10], [11], [12] | |||
| 2 | MTR105 | Drug Info | [13] | |||
| 3 | VAS-203 | Drug Info | [14] | |||
| 4 | L-NIL | Drug Info | [16] | |||
| 5 | (5-Imino-[1,4]thiazepan-3-yl)-methanol | Drug Info | [17] | |||
| 6 | (5S,6R)-[Octahydro-quinolin-(2E)-ylidene]amine | Drug Info | [18] | |||
| 7 | (5S,6S)-[Octahydro-quinolin-(2E)-ylidene]amine | Drug Info | [18] | |||
| 8 | (6r,1'r,2's)-5,6,7,8 Tetrahydrobiopterin | Drug Info | [19] | |||
| 9 | (S)-2-Amino-5-(N-methyl-guanidino)-pentanoic acid | Drug Info | [20] | |||
| 10 | (S)-3-Propyl-[1,4]thiazepan-(5E)-ylideneamine | Drug Info | [17] | |||
| 11 | (S)-6-Amino-2-(2-imino-ethylamino)-hexanoic acid | Drug Info | [17] | |||
| 12 | 1,2,4-Triazole-Carboxamidine | Drug Info | [19] | |||
| 13 | 1-(2-amino-benzothiazol-5-yl)-2-ethyl-isothiourea | Drug Info | [21] | |||
| 14 | 1-(2-amino-benzothiazol-6-yl)-2-ethyl-isothiourea | Drug Info | [21] | |||
| 15 | 1400W | Drug Info | [22] | |||
| 16 | 2,4-Diamino-6-Phenyl-5,6,7,8,-Tetrahydropteridine | Drug Info | [22] | |||
| 17 | 2-amino-4-methylpyridine | Drug Info | [23] | |||
| 18 | 2-Amino-5-(N-nitro-guanidino)-pentanoic acid | Drug Info | [20] | |||
| 19 | 2-Aminothiazoline | Drug Info | [19], [24] | |||
| 20 | 2-Methyl-2,4-Pentanediol | Drug Info | [19] | |||
| 21 | 2-Methyl-[1,4]thiazepan-(5E)-ylideneamine | Drug Info | [17] | |||
| 22 | 2-Propanol, Isopropanol | Drug Info | [19] | |||
| 23 | 3,4-Dimethyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 24 | 3-Bromo-1H-indazole-7-carbonitrile | Drug Info | [25] | |||
| 25 | 3-bromo-7-nitro-1H-indazole | Drug Info | [19], [25] | |||
| 26 | 3-Ethyl-[1,4]thiazepan-(5E)-ylideneamine | Drug Info | [17] | |||
| 27 | 3-Methyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 28 | 3-Methyl-[1,4]thiazepan-(5E)-ylideneamine | Drug Info | [17] | |||
| 29 | 4,5-Dimethyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 30 | 4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine | Drug Info | [26] | |||
| 31 | 4-Ethyl-5-methyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 32 | 4-Ethyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 33 | 4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine | Drug Info | [26] | |||
| 34 | 4-Methyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine | Drug Info | [26] | |||
| 35 | 4-methyl-6-propylpyridin-2-amine | Drug Info | [23] | |||
| 36 | 4-Methyl-piperidin-(2E)-ylideneamine | Drug Info | [18] | |||
| 37 | 4-Methyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 38 | 5,6-Cyclic-Tetrahydropteridine | Drug Info | [27] | |||
| 39 | 5-Ethyl-3-methyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 40 | 5-Ethyl-4-methyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 41 | 5-Methyl-pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 42 | 5-Nitroindazole | Drug Info | [19] | |||
| 43 | 6-(2-Fluoropropyl)-4-methylpyridin-2-amine | Drug Info | [28] | |||
| 44 | 6-(3-Fluoropropyl)-4-methylpyridin-2-amine | Drug Info | [28] | |||
| 45 | 6-isobutyl-4-methylpyridin-2-amine | Drug Info | [28] | |||
| 46 | 6-Nitroindazole | Drug Info | [19] | |||
| 47 | 6s-5,6,7,8-Tetrahydrobiopterin | Drug Info | [19] | |||
| 48 | 7-(2-Nitro-ethyl)-azepan-(2Z)-ylideneamine | Drug Info | [29] | |||
| 49 | 7-Methyl-[1,4]thiazepan-(5E)-ylideneamine | Drug Info | [17] | |||
| 50 | 7-nitro-1H-indazole | Drug Info | [19] | |||
| 51 | 7-Nitroindazole-2-Carboxamidine | Drug Info | [22] | |||
| 52 | Acetate Ion | Drug Info | [19] | |||
| 53 | AP-Cav | Drug Info | [30] | |||
| 54 | Azepan-(2Z)-ylideneamine | Drug Info | [29] | |||
| 55 | Cacodylate Ion | Drug Info | [19] | |||
| 56 | Ethylisothiourea | Drug Info | [19] | |||
| 57 | Heme | Drug Info | [22] | |||
| 58 | Hexahydro-cyclopenta[c]pyrrol-(1Z)-ylideneamine | Drug Info | [24] | |||
| 59 | Hydroxydimethylarsine Oxide | Drug Info | [19] | |||
| 60 | L-2-Amino-4-(Guanidinooxy)Butyric Acid | Drug Info | [19] | |||
| 61 | L-Homoarginine | Drug Info | [19] | |||
| 62 | L-NIO | Drug Info | [27], [31] | |||
| 63 | N,N-dimethylarginine | Drug Info | [32] | |||
| 64 | N-(5-Amino-6-oxo-heptyl)-acetamidine | Drug Info | [24] | |||
| 65 | N-(Chlorophenyl)-N'-Hydroxyguanidine | Drug Info | [22] | |||
| 66 | N-omega-allyl-L-arginine | Drug Info | [31] | |||
| 67 | N-Omega-Hydroxy-L-Arginine | Drug Info | [19] | |||
| 68 | N-omega-propargyl-L-arginine | Drug Info | [31] | |||
| 69 | N1,N14-Bis((S-Methyl)Isothioureido)Tetradecane | Drug Info | [19] | |||
| 70 | N5-(1-iminobut-3-enyl)-L-ornithine | Drug Info | [31] | |||
| 71 | N5-(1-iminobutyl)-L-ornithine | Drug Info | [31] | |||
| 72 | N5-(1-iminopropyl)-L-ornithine | Drug Info | [31] | |||
| 73 | Nitroarginine | Drug Info | [33] | |||
| 74 | Piperidin-(2E)-ylideneamine | Drug Info | [18] | |||
| 75 | Pyrrolidin-(2Z)-ylideneamine | Drug Info | [24] | |||
| 76 | S-(Dimethylarsenic)Cysteine | Drug Info | [19] | |||
| 77 | S-Ethyl-N-Phenyl-Isothiourea | Drug Info | [22] | |||
| 78 | S-Isopropyl-Isothiourea | Drug Info | [19] | |||
| 79 | Se-Ethyl-Isoselenourea | Drug Info | [19] | |||
| 80 | THIOCITRULLINE | Drug Info | [34] | |||
| 81 | [1,3]Oxazinan-(2E)-ylideneamine | Drug Info | [20] | |||
| 82 | [1,3]Thiazinan-(2E)-ylideneamine | Drug Info | [20] | |||
| 83 | [1,4]Oxazepan-(3E)-ylideneamine | Drug Info | [17] | |||
| 84 | [1,4]Thiazepan-(3E)-ylideneamine | Drug Info | [17] | |||
| 85 | [1,4]Thiazepan-(5E)-ylideneamine | Drug Info | [17] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Arginine | Ligand Info | |||||
| Structure Description | Structure of human endothelial nitric oxide synthase heme domain with L-Arg bound | PDB:4D1O | ||||
| Method | X-ray diffraction | Resolution | 1.82 Å | Mutation | No | [35] |
| PDB Sequence |
FPRVKNWEVG
77 SITYDTLSAQ87 AQQDGPCTPR97 RCLGSLVFPA119 PEQLLSQARD129 FINQYYSSIK 139 RSGSQAHEQR149 LQEVEAEVAA159 TGTYQLRESE169 LVFGAKQAWR179 NAPRCVGRIQ 189 WGKLQVFDAR199 DCRSAQEMFT209 YICNHIKYAT219 NRGNLRSAIT229 VFPQRCPGRG 239 DFRIWNSQLV249 RYAGYRQQDG259 SVRGDPANVE269 ITELCIQHGW279 TPGNGRFDVL 289 PLLLQAPDEP299 PELFLLPPEL309 VLEVPLEHPT319 LEWFAALGLR329 WYALPAVSNM 339 LLEIGGLEFP349 AAPFSGWYMS359 TEIGTRNLCD369 PHRYNILEDV379 AVCMDLDTRT 389 TSSLWKDKAA399 VEINVAVLHS409 YQLAKVTIVD419 HHAATASFMK429 HLENEQKARG 439 GCPADWAWIV449 PPISGSLTPV459 FHQEMVNYFL469 SPAFRYQPDP479 W |
|||||
|
|
||||||
| Ligand Name: Chlorzoxazone | Ligand Info | |||||
| Structure Description | human endothelial nitric oxide synthase with chlorzoxazone bound | PDB:1M9J | ||||
| Method | X-ray diffraction | Resolution | 2.43 Å | Mutation | No | [36] |
| PDB Sequence |
KFPRVKNWEV
76 GSITYDTLSA86 QAQQDGPCTP96 RRCLGSLVFP120 EQLLSQARDF130 INQYYSSIKR 140 SGSQAHEQRL150 QEVEAEVAAT160 GTYQLRESEL170 VFGAKQAWRN180 APRCVGRIQW 190 GKLQVFDARD200 CRSAQEMFTY210 ICNHIKYATN220 RGNLRSAITV230 FPQRCPGRGD 240 FRIWNSQLVR250 YAGYRQQDGS260 VRGDPANVEI270 TELCIQHGWT280 PGNGRFDVLP 290 LLLQAPDEPP300 ELFLLPPELV310 LEVPLEHPTL320 EWFAALGLRW330 YALPAVSNML 340 LEIGGLEFPA350 APFSGWYMST360 EIGTRNLCDP370 HRYNILEDVA380 VCMDLDTRTT 390 SSLWKDKAAV400 EINVAVLHSY410 QLAKVTIVDH420 HAATASFMKH430 LENEQKARGG 440 CPADWAWIVP450 PISGSLTPVF460 HQEMVNYFLS470 PAFRYQPDPW480 |
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Arginine biosynthesis | hsa00220 | Affiliated Target |

|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Arginine and proline metabolism | hsa00330 | Affiliated Target |

|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| cGMP-PKG signaling pathway | hsa04022 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
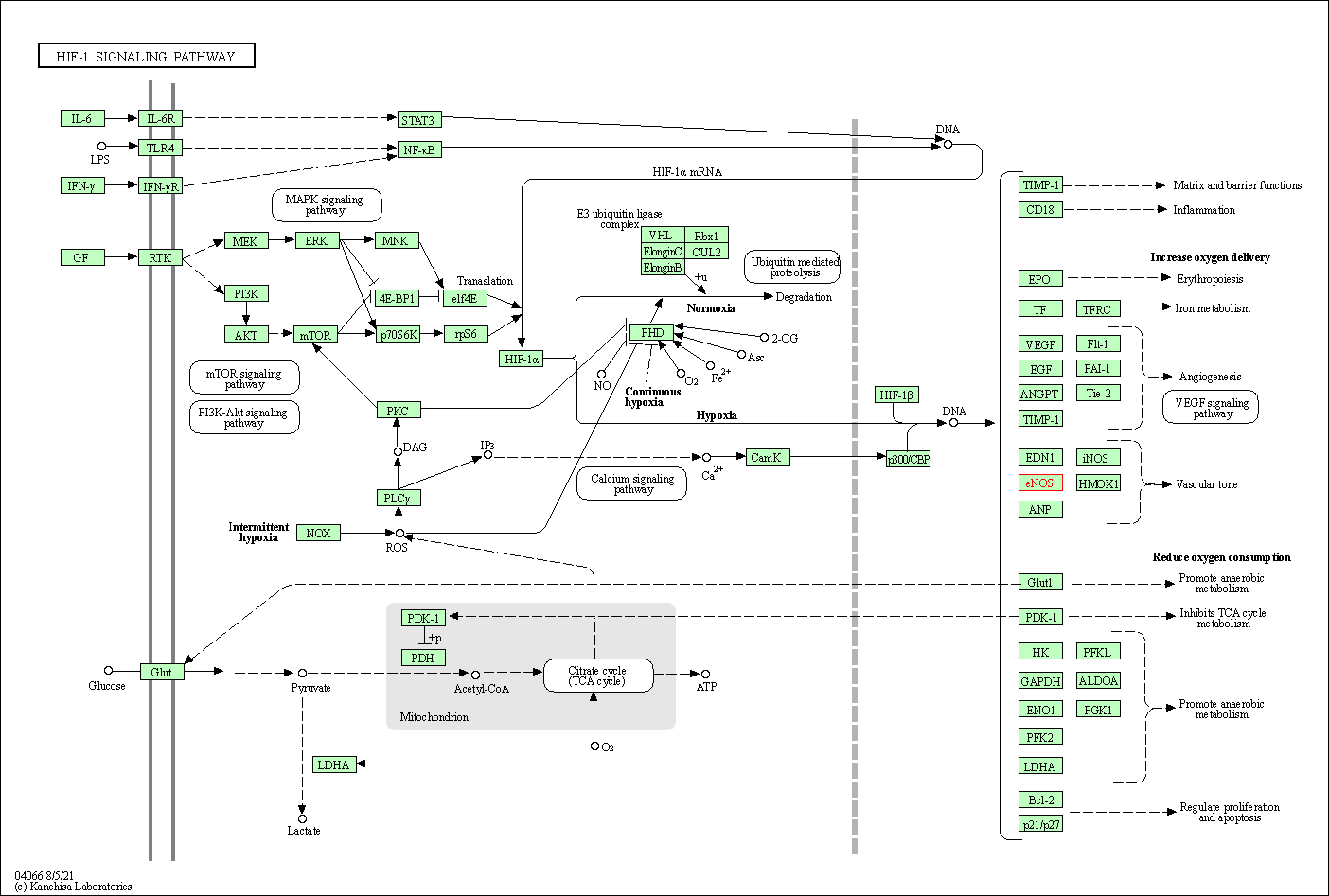
| HIF-1 signaling pathway | hsa04066 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
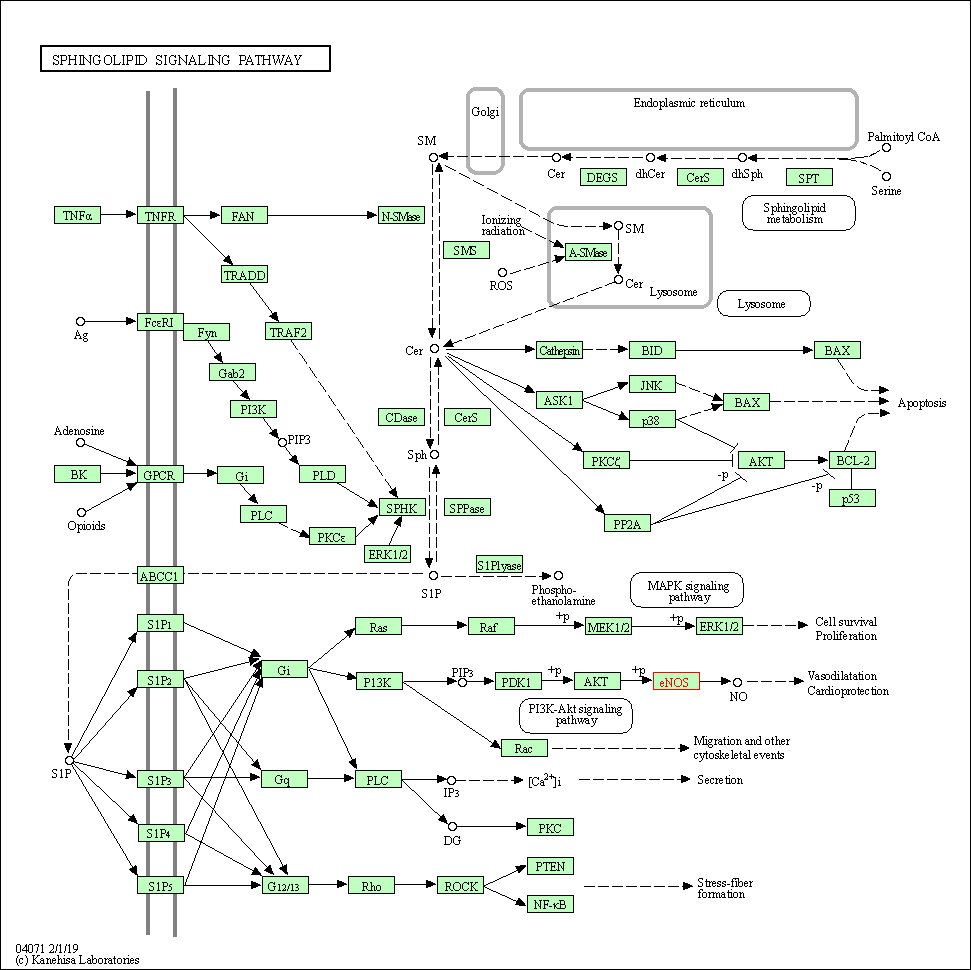
| Sphingolipid signaling pathway | hsa04071 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
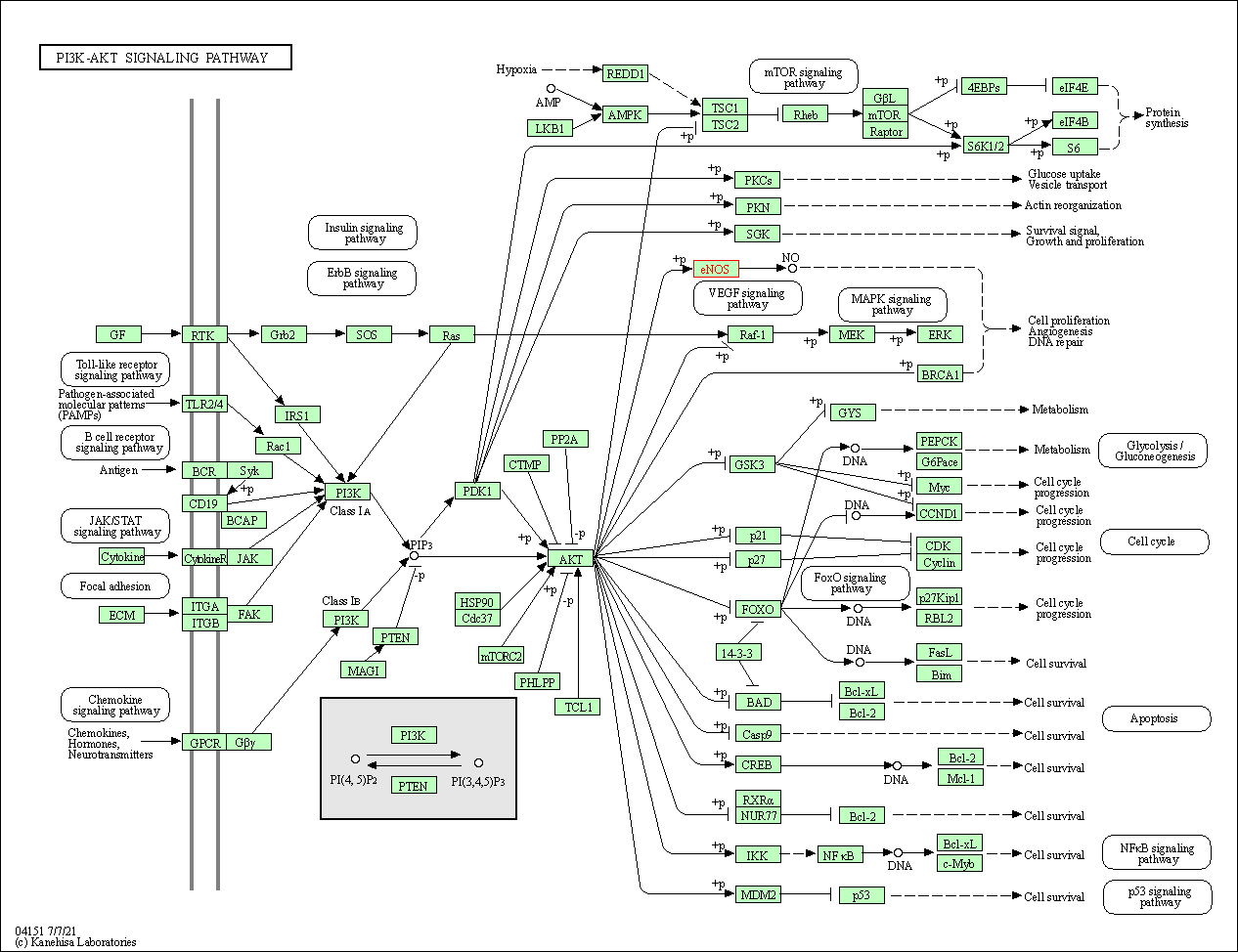
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
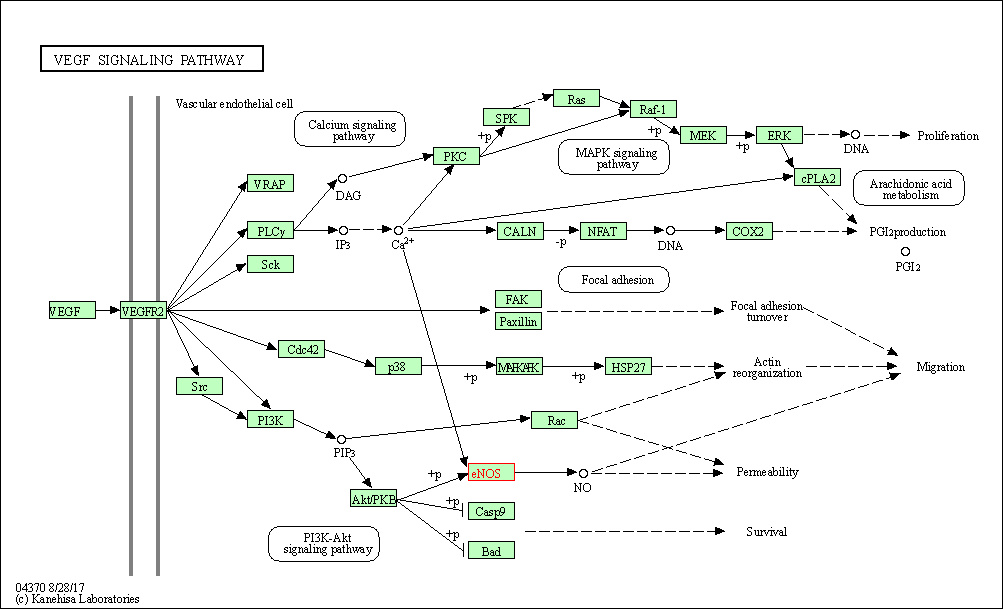
| VEGF signaling pathway | hsa04370 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
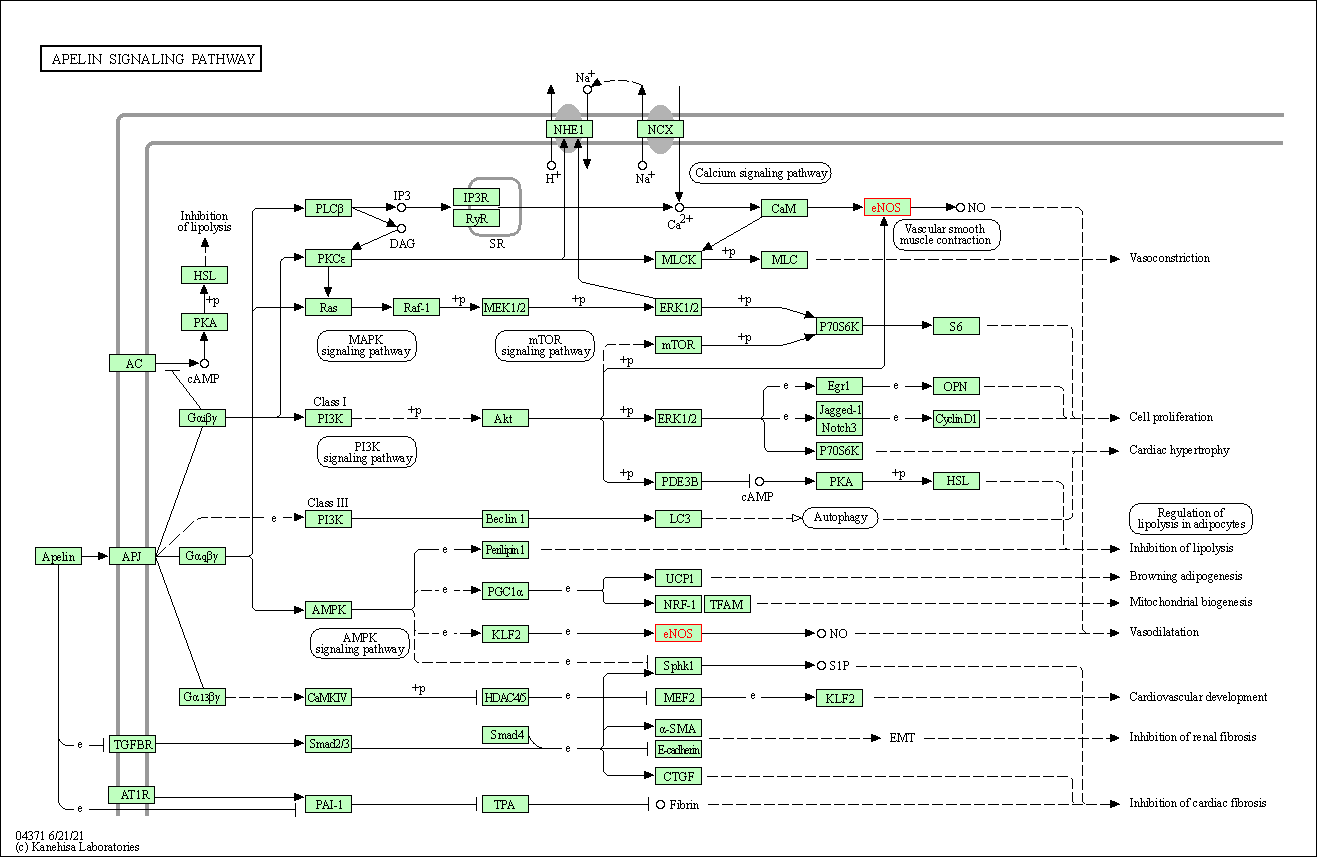
| Apelin signaling pathway | hsa04371 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
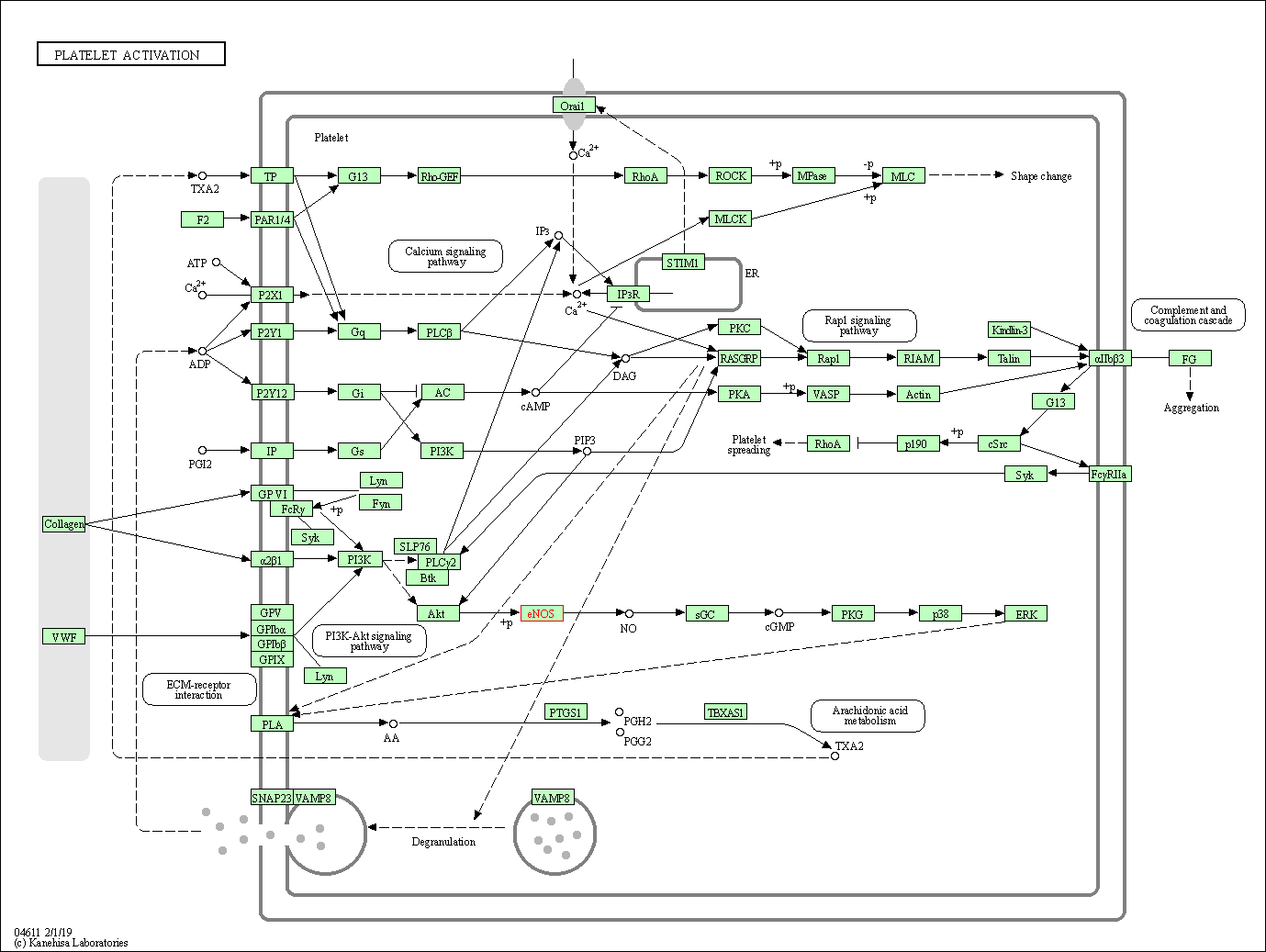
| Platelet activation | hsa04611 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
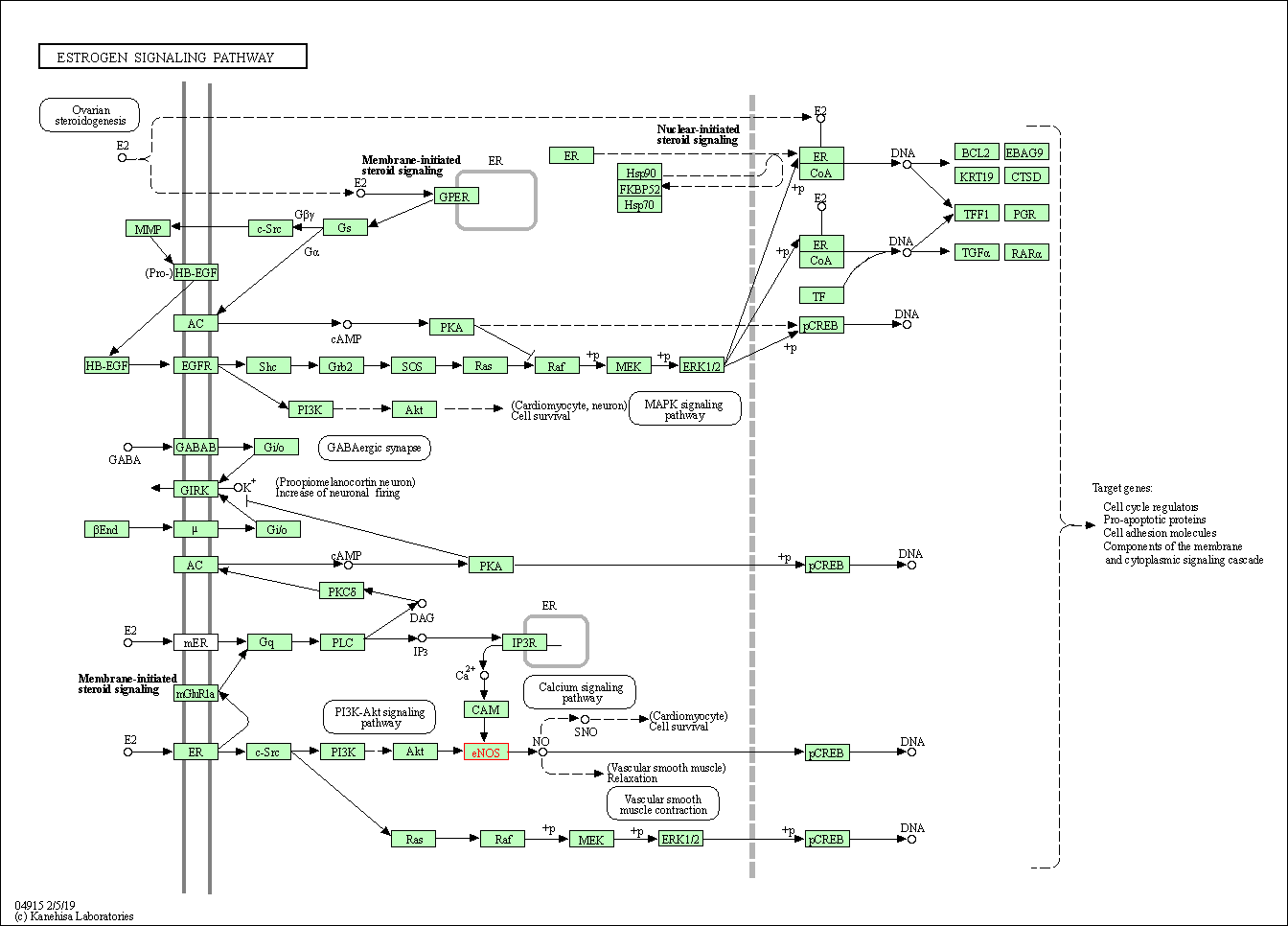
| Estrogen signaling pathway | hsa04915 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
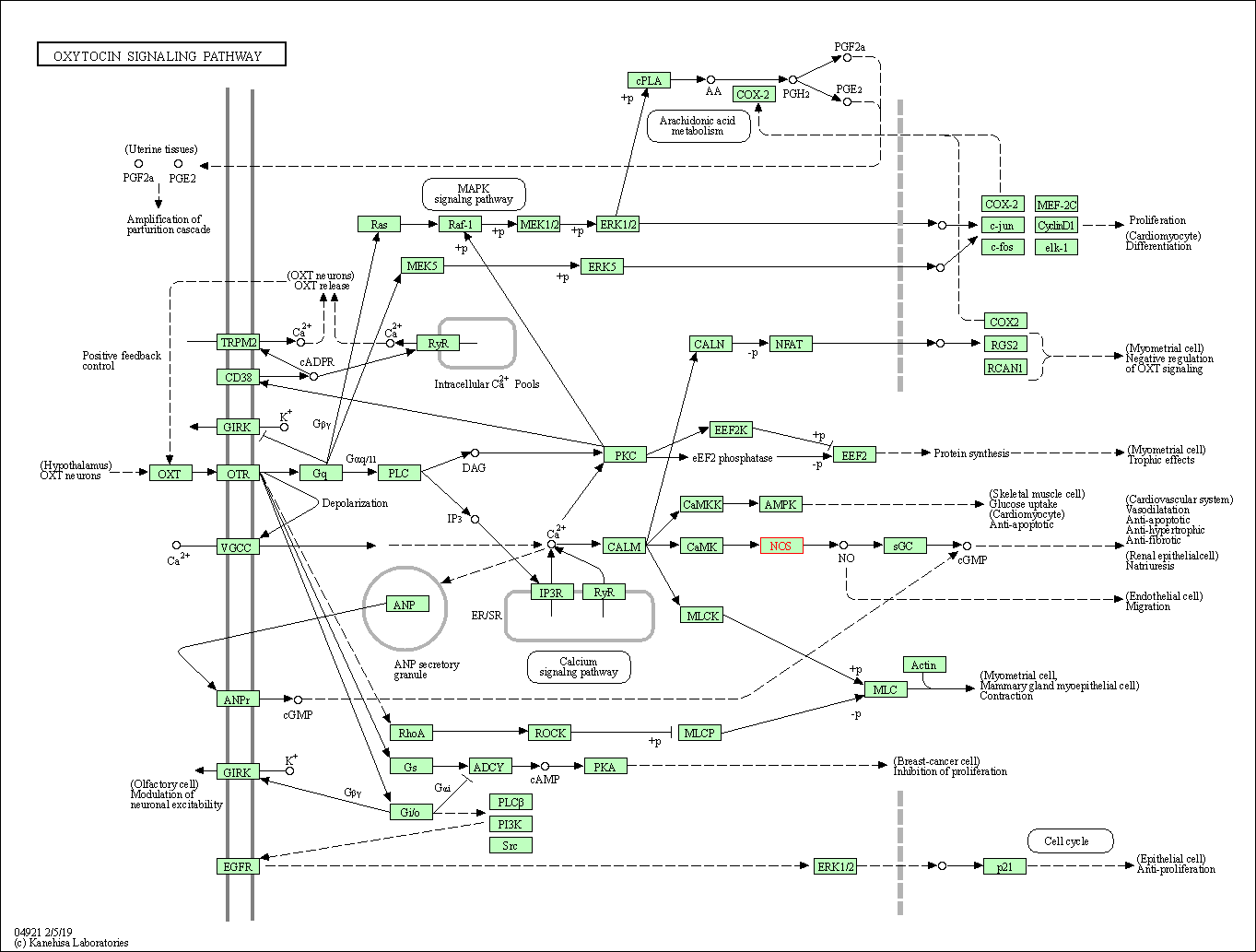
| Oxytocin signaling pathway | hsa04921 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Relaxin signaling pathway | hsa04926 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 21 | Degree centrality | 2.26E-03 | Betweenness centrality | 4.64E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.54E-01 | Radiality | 1.44E+01 | Clustering coefficient | 1.48E-01 |
| Neighborhood connectivity | 4.60E+01 | Topological coefficient | 7.30E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007 Apr 18;297(15):1657-66. | |||||
| REF 2 | ClinicalTrials.gov (NCT00112281) A Study of the Safety and Efficacy of Nitric Oxide Reduction in Patients With Cardiogenic Shock After a Heart Attack. U.S. National Institutes of Health. | |||||
| REF 3 | ClinicalTrials.gov (NCT01116427) A Cooperative Clinical Study of Abatacept in Multiple Sclerosis. U.S. National Institutes of Health. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5213). | |||||
| REF 5 | Inhibition of nitric oxide synthase by L-NAME speeds phase II pulmonary .VO2 kinetics in the transition to moderate-intensity exercise in man. J Physiol. 2003 Oct 1;552(Pt 1):265-72. | |||||
| REF 6 | ClinicalTrials.gov (NCT00482287) Pharmacokinetics and Pharmacodynamics of MTR105 in Hypotensive Cardiac Surgery Patients. U.S. National Institutes of Health. | |||||
| REF 7 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023224) | |||||
| REF 8 | A Phase I study of the transplantation of genetically marked autologous bone marrow stromal cells. Hum Gene Ther. 1998 Mar 1;9(4):591-600. | |||||
| REF 9 | CenterWatch. Drugs in Clinical Trials Database. CenterWatch. 2008. | |||||
| REF 10 | Counter-regulation by atorvastatin of gene modulations induced by L-NAME hypertension is associated with vascular protection. Vascul Pharmacol. 2009 Oct;51(4):253-61. | |||||
| REF 11 | Enhanced pulmonary expression of the TrkB neurotrophin receptor in hypoxic rats is associated with increased acetylcholine-induced airway contracti... Acta Physiol (Oxf). 2009 Nov;197(3):253-64. | |||||
| REF 12 | L-NAME causes antinociception by stimulation of the arginine-NO-cGMP pathway. Mediators Inflamm. 2000;9(1):25-30. | |||||
| REF 13 | Nitric oxide synthase inhibitor (MTR-105) during open-heart surgery. A pilot double-blind placebo-controlled study of hemodynamic effects and safety. Cardiology. 2008;111(3):181-7. | |||||
| REF 14 | Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: a placebo-controlled randomized Phase IIa trial (NOSTRA). J Neurotrauma. 2014 Oct 1;31(19):1599-606. | |||||
| REF 15 | Feasibility of using autologous transplantation to evaluate hematopoietic stem cell-based gene therapy strategies in transgenic mouse models of human disease. Mol Ther. 2002 Sep;6(3):422-8. | |||||
| REF 16 | Discovery of a series of aminopiperidines as novel iNOS inhibitors. Bioorg Med Chem Lett. 2008 Jan 1;18(1):336-43. | |||||
| REF 17 | Synthesis of analogs of (1,4)-3- and 5-imino oxazepane, thiazepane, and diazepane as inhibitors of nitric oxide synthases. Bioorg Med Chem Lett. 2004 Dec 6;14(23):5907-11. | |||||
| REF 18 | Bicyclic amidine inhibitors of nitric oxide synthase: discovery of perhydro-iminopyrindine and perhydro-iminoquinoline as potent, orally active inh... Bioorg Med Chem Lett. 2005 Apr 15;15(8):1997-2001. | |||||
| REF 19 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 20 | 2-Iminopiperidine and other 2-iminoazaheterocycles as potent inhibitors of human nitric oxide synthase isoforms. J Med Chem. 1996 Feb 2;39(3):669-72. | |||||
| REF 21 | Novel 2-aminobenzothiazoles as selective neuronal nitric oxide synthase inhibitors. Bioorg Med Chem Lett. 2007 May 1;17(9):2540-4. | |||||
| REF 22 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 23 | Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat Chem Biol. 2008 Nov;4(11):700-7. | |||||
| REF 24 | Evaluation of pyrrolidin-2-imines and 1,3-thiazolidin-2-imines as inhibitors of nitric oxide synthase. Bioorg Med Chem Lett. 2004 Sep 6;14(17):4539-44. | |||||
| REF 25 | Inhibitory effects of a series of 7-substituted-indazoles toward nitric oxide synthases: particular potency of 1H-indazole-7-carbonitrile. Bioorg Med Chem. 2008 Jun 1;16(11):5962-73. | |||||
| REF 26 | Design and synthesis of orally bioavailable inhibitors of inducible nitric oxide synthase. Part 1: synthesis and biological evaluation of dihydropy... Bioorg Med Chem Lett. 2002 Sep 2;12(17):2291-4. | |||||
| REF 27 | DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 2011 Jan;39(Database issue):D1035-41. | |||||
| REF 28 | Design and synthesis of 2-amino-4-methylpyridine analogues as inhibitors for inducible nitric oxide synthase and in vivo evaluation of [18F]6-(2-fl... J Med Chem. 2009 Apr 23;52(8):2443-53. | |||||
| REF 29 | Selective heterocyclic amidine inhibitors of human inducible nitric oxide synthase. Bioorg Med Chem Lett. 2001 Oct 8;11(19):2651-3. | |||||
| REF 30 | Endothelial nitric oxide synthase: the Cinderella of inflammation Trends Pharmacol Sci. 2003 Feb;24(2):91-5. | |||||
| REF 31 | Structure-activity relationship of novel and known inhibitors of human dimethylarginine dimethylaminohydrolase-1: alkenyl-amidines as new leads. Bioorg Med Chem. 2008 Dec 15;16(24):10205-9. | |||||
| REF 32 | Homocysteine and asymmetric dimethylarginine (ADMA): biochemically linked but differently related to vascular disease in chronic kidney disease. Clin Chem Lab Med. 2007;45(12):1683-7. | |||||
| REF 33 | Crystal structure of nitric oxide synthase bound to nitro indazole reveals a novel inactivation mechanism. Biochemistry. 2001 Nov 13;40(45):13448-55. | |||||
| REF 34 | Evaluation of 3-substituted arginine analogs as selective inhibitors of human nitric oxide synthase isozymes. Bioorg Med Chem Lett. 2005 Jun 2;15(11):2881-5. | |||||
| REF 35 | Structures of human constitutive nitric oxide synthases. Acta Crystallogr D Biol Crystallogr. 2014 Oct;70(Pt 10):2667-74. | |||||
| REF 36 | Conformational changes in nitric oxide synthases induced by chlorzoxazone and nitroindazoles: crystallographic and computational analyses of inhibitor potency. Biochemistry. 2002 Nov 26;41(47):13915-25. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

