Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0J7EH
|
|||
| Former ID |
DCL000030
|
|||
| Drug Name |
ACCLAIM
|
|||
| Synonyms |
Depon; Excel; Furore; PUMA; Whip; Fenoxaprop ethyl; Fenoxaprop ethyl ester; Fenoxaprop ethyl ester [ANSI]; Tiller Herbicide; HOE 33171; FENOXAPROP-ETHYL; HOE-A 25-01; Ethyl 2-(4-((6-chlorobenzoxazol-2-yl)oxy)phenoxy)propionate; Ethyl 2-[4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy]propanoate; Ethyl (D+)-2-(4-(6-chlor-2-benzoxazolyloxy)phenoxy)propanoate; Ethyl (D+)-2-(4-(6-chlor-2-benzoxazolyloxy)phenoxy)propanoate [French]; Ethyl 2-(4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy)propanoate; Ethyl 2-{4-[(6-chloro-1,3-benzoxazol-2-yl)oxy]phenoxy}propanoate; Ethyl-2-((4-(6-chloro-2-benzoxazolyloxy))-phenoxy)propionate; Ethyl (+-)-2-(4-(6-chloro-2-benzoxazolyloxy)phenoxy)propanoate; Propanoic acid, 2-(4-((6-chloro-2-benzoxazolyl)oxy)phenoxy)-, ethyl ester; (+/-)-2-(4-((6-Chloro-2-benzoxazolyl)oxy)phenoxy)propanoic acid ethyl ester; 2-(4-((6-Chloro-2-benzoxazolylen)oxy)phenoxy)propanoic acid, ethyl ester
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Angina pectoris [ICD-11: BA40] | Phase 2 | [1] | |
| Coronary artery disease [ICD-11: BA80; ICD-10: I25.1] | Phase 2 | [1] | ||
| Company |
Angiogenix
|
|||
| Structure |
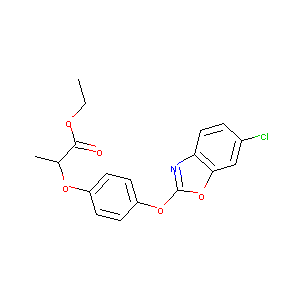 |
Download2D MOL |
||
| Formula |
C18H16ClNO5
|
|||
| Canonical SMILES |
CCOC(=O)C(C)OC1=CC=C(C=C1)OC2=NC3=C(O2)C=C(C=C3)Cl
|
|||
| InChI |
1S/C18H16ClNO5/c1-3-22-17(21)11(2)23-13-5-7-14(8-6-13)24-18-20-15-9-4-12(19)10-16(15)25-18/h4-11H,3H2,1-2H3
|
|||
| InChIKey |
PQKBPHSEKWERTG-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 66441-23-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
13207, 6755668, 8180468, 14926007, 34712873, 47919709, 47994668, 48425882, 50022219, 50113104, 50113105, 57313163, 77630638, 92251428, 103211554, 104354210, 121279343, 124893478, 125355783, 126685344, 128953307, 131322999, 134338741, 135007684, 135013078, 137015951, 140371212, 144211483, 152035211, 162180818, 162252035, 162305047, 164756949, 175426962, 176286330, 176306152, 179316689, 184556555, 206246298, 223439687, 223678202, 226437562, 250113001, 252270257, 252369246, 252448729
|
|||
| ChEBI ID |
CHEBI:5008
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01116427) A Cooperative Clinical Study of Abatacept in Multiple Sclerosis. U.S. National Institutes of Health. | |||
| REF 2 | CenterWatch. Drugs in Clinical Trials Database. CenterWatch. 2008. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

