Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0X1RQ
|
|||
| Former ID |
DNCL001790
|
|||
| Drug Name |
MTR105
|
|||
| Synonyms |
UNII-CTD25O6OBI; CTD25O6OBI; MTR 105; S-Ethylisothiuronium diethyl phosphate; 21704-46-1; Carbamimidothioic acid, ethyl ester, mono(diethyl phospate); Phosphoric acid, diethyl ester, compd. with 2-ethyl-2-thiopseudourea (1:1); Difetur; AC1Q6SNE; AC1L4PKY; CTK4E7529; nitric oxide synthase inhibi-tors; ethyl carbamimidothioate- diethyl hydrogen phosphate(1:1); MTR-105; DTXSID60176093; AKOS030611139; LS-107705; diethyl hydrogen phosphate; Carbamimidothioic acid, ethyl ester, diethyl phosphate (1
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypotension [ICD-11: BA20-BA21] | Phase 2 | [1] | |
| Company |
Meditor Pharmaceuticals
|
|||
| Structure |
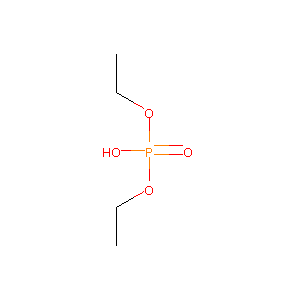 |
Download2D MOL |
||
| Formula |
C7H19N2O4PS
|
|||
| Canonical SMILES |
CCOP(=O)(O)OCC.CCSC(=N)N
|
|||
| InChI |
1S/C4H11O4P.C3H8N2S/c1-3-7-9(5,6)8-4-2;1-2-6-3(4)5/h3-4H2,1-2H3,(H,5,6);2H2,1H3,(H3,4,5)
|
|||
| InChIKey |
CSYSULGPHGCBQD-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 21704-46-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00482287) Pharmacokinetics and Pharmacodynamics of MTR105 in Hypotensive Cardiac Surgery Patients. U.S. National Institutes of Health. | |||
| REF 2 | Nitric oxide synthase inhibitor (MTR-105) during open-heart surgery. A pilot double-blind placebo-controlled study of hemodynamic effects and safety. Cardiology. 2008;111(3):181-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

