Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0G1IV
|
|||
| Former ID |
DIB014702
|
|||
| Drug Name |
Testogen TDS
|
|||
| Synonyms |
OriTex; OriTex); TDS-testosterone; Testosterone (oral, testosterone deficiency), Clarus; Testosterone (transdermal, TDS), TransDermal Technologies
Click to Show/Hide
|
|||
| Indication | Hypogonadism [ICD-11: 5A61.0; ICD-9: 257.2] | Phase 2 | [1] | |
| Company |
TransDermal Technologies Inc; Clarus Therapeutics Inc
|
|||
| Structure |
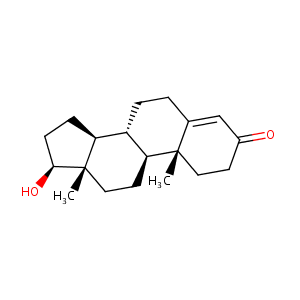 |
Download2D MOL |
||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01894308) A Dose Ranging Study to Examine Testagen TDS-Testosterone 5%. U.S. National Institutes of Health. | |||
| REF 2 | Evidence for a role of testosterone-androgen receptor interactions in mediating masculine sexual behavior in male rats. Endocrinology. 1989 Feb;124(2):618-26. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

