Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0G2BS
|
|||
| Former ID |
DNC004850
|
|||
| Drug Name |
2-(4-Methyl-piperazin-1-yl)-quinoline
|
|||
| Synonyms |
N-methylquipazine; 2-(4-methylpiperazin-1-yl)quinoline; UNII-0YV1ZIR6S0; 0YV1ZIR6S0; CHEMBL288591; CHEBI:64164; quinoline, 2-(4-methyl-1-piperazinyl)-; 2-(4-Methyl-piperazin-1-yl)-quinoline; Tocris-0566; Lopac-Q-107; Biomol-NT_000084; AC1L1JF3; Oprea1_654246; Lopac0_001000; SCHEMBL606721; BPBio1_001081; DTXSID8043731; CTK6I3065; HOMWNUXPSJQSSU-UHFFFAOYSA-N; MolPort-006-384-975; ZINC403653; 2-(4-Methylpiperazinyl)-quinoline; 1-(2-Quinolyl)-4-methylpiperazine; STK362919; BDBM50053631; AKOS005453926; MCULE-4786527390; CCG-205080
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
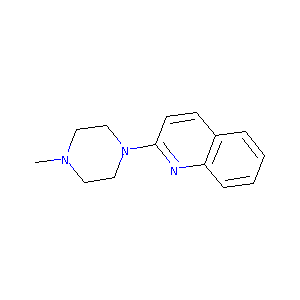 |
Download2D MOL |
||
| Formula |
C14H17N3
|
|||
| Canonical SMILES |
CN1CCN(CC1)C2=NC3=CC=CC=C3C=C2
|
|||
| InChI |
1S/C14H17N3/c1-16-8-10-17(11-9-16)14-7-6-12-4-2-3-5-13(12)15-14/h2-7H,8-11H2,1H3
|
|||
| InChIKey |
HOMWNUXPSJQSSU-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 28614-26-8
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:64164
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 3A receptor (HTR3A) | Target Info | Inhibitor | [2] |
| 5-HT 3B receptor (HTR3B) | Target Info | Inhibitor | [2] | |
| Histamine H3 receptor (H3R) | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Serotonergic synapse | |||
| Neuroactive ligand-receptor interaction | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | 5HT3 type receptor mediated signaling pathway | |||
| Reactome | Ligand-gated ion channel transport | |||
| Histamine receptors | ||||
| G alpha (i) signalling events | ||||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Iron uptake and transport | ||||
| Monoamine Transport | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2-(4-alkylpiperazin-1-yl)quinolines as a new class of imidazole-free histamine H3 receptor antagonists. J Med Chem. 2005 Jan 13;48(1):306-11. | |||
| REF 2 | Novel potent and selective central 5-HT3 receptor ligands provided with different intrinsic efficacy. 2. Molecular basis of the intrinsic efficacy ... J Med Chem. 1999 May 6;42(9):1556-75. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

