Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0J5OV
|
|||
| Former ID |
DNC000417
|
|||
| Drug Name |
Chebulagic acid
|
|||
| Synonyms |
Chebulagic acid; 23094-71-5; NSC 342674; AC1L2HS4; beta-D-Glucopyranose, cyclic 3,6-(4,4',5,5',6,6'-hexahydroxy(1,1'-biphenyl)-2,2'-dicarboxylate) 1-(3,4,5-trihydroxybenzoate), cyclic 2-5:4-1-ester with (5-carboxy-3,4-dihydro-3,7,8-trihydroxy-2-oxo-2H-1-benzopyran-4-yl)butanedioic acid, stereoisomer
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
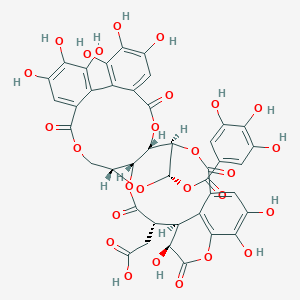 |
Download2D MOL
|
||
| Formula |
C41H30O27
|
|||
| Canonical SMILES |
C1C2C3C(C(C(O2)OC(=O)C4=CC(=C(C(=C4)O)O)O)OC(=O)C5=CC(=C(C6=C5C(C(C(=O)O3)CC(=O)O)C(C(=O)O6)O)O)O)OC(=O)C7=CC(=C(C(=C7C8=C(C(=C(C=C8C(=O)O1)O)O)O)O)O)O
|
|||
| InChI |
1S/C41H30O27/c42-13-1-8(2-14(43)24(13)49)35(56)68-41-34-33-31(64-39(60)12(6-19(47)48)22-23-11(38(59)67-34)5-17(46)27(52)32(23)65-40(61)30(22)55)18(63-41)7-62-36(57)9-3-15(44)25(50)28(53)20(9)21-10(37(58)66-33)4-16(45)26(51)29(21)54/h1-5,12,18,22,30-31,33-34,41-46,49-55H,6-7H2,(H,47,48)/t12-,18+,22-,30-,31+,33-,34+,41-/m0/s1
|
|||
| InChIKey |
HGJXAVROWQLCTP-YABCKIEDSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:3583
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 5-lipoxygenase (5-LOX) | Target Info | Inhibitor | [1] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Vitamin D Receptor Pathway | |||
| Arachidonic acid metabolism | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J Ethnopharmacol. 2009 Jul 30;124(3):506-12. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

