Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0N1SU
|
|||
| Former ID |
DCL000302
|
|||
| Drug Name |
Licofelone
|
|||
| Synonyms |
LCF; Licofelone [INN]; ML 3000; ML-3000; [6-(4-CHLOROPHENYL)-2,2-DIMETHYL-7-PHENYL-2,3-DIHYDRO-1H-PYRROLIZIN-5-YL]ACETIC ACID; (2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl)acetic acid; (6-(4-Chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1H-pyrrolizin-5-yl)acetic acid; 2,2-Dimethyl-6-(4-chlorophenyl-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl)acetic acid; 2,3-Dihydro-6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-1H-pyrrolizine-5-acetic acid; 2-[2-(4-chlorophenyl)-6,6-dimethyl-1-phenyl-5,7-dihydropyrrolizin-3-yl]acetic acid; 6-(4-Chlorophenyl)-2,3-dihydro-2,2-dimethyl-7-phenyl-1H-pyrrolizine-5-acetic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Osteoarthritis [ICD-11: FA00-FA05; ICD-9: 715] | Discontinued in Phase 1 | [1] | |
| Company |
Merckle GmbH
|
|||
| Structure |
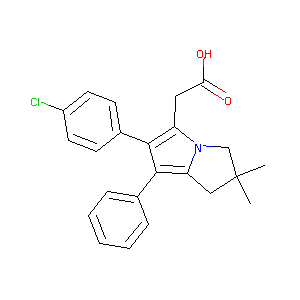 |
Download2D MOL |
||
| Formula |
C23H22ClNO2
|
|||
| Canonical SMILES |
CC1(CC2=C(C(=C(N2C1)CC(=O)O)C3=CC=C(C=C3)Cl)C4=CC=CC=C4)C
|
|||
| InChI |
1S/C23H22ClNO2/c1-23(2)13-19-22(15-6-4-3-5-7-15)21(16-8-10-17(24)11-9-16)18(12-20(26)27)25(19)14-23/h3-11H,12-14H2,1-2H3,(H,26,27)
|
|||
| InChIKey |
UAWXGRJVZSAUSZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 156897-06-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
6436380, 7888616, 10243273, 12014886, 14829304, 29311030, 50040041, 50700417, 53788587, 57344354, 103243188, 103945934, 104378798, 125333743, 126645607, 126648934, 129342134, 131300446, 134338958, 135088300, 135692433, 137116560, 137275875, 142116170, 144072480, 162172234, 162476140, 163884604, 164228207, 164761582, 175426859, 184826912, 196105884, 198972330, 204370171, 223392829, 223705238, 223909705, 226588726, 251882412, 252215695, 252450609
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 5-lipoxygenase (5-LOX) | Target Info | Inhibitor | [2], [3], [4], [5] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Vitamin D Receptor Pathway | |||
| Arachidonic acid metabolism | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003927) | |||
| REF 2 | Cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) selectivity of COX inhibitors. Prostaglandins Leukot Essent Fatty Acids. 2008 Feb;78(2):99-108. | |||
| REF 3 | Activity and potential role of licofelone in the management of osteoarthritis. Clin Interv Aging. 2007;2(1):73-9. | |||
| REF 4 | Licofelone, a dual COX/5-LOX inhibitor, induces apoptosis in HCA-7 colon cancer cells through the mitochondrial pathway independently from its abil... Carcinogenesis. 2008 Feb;29(2):371-80. | |||
| REF 5 | Licofelone (ML-3000), a dual inhibitor of 5-lipoxygenase and cyclooxygenase, reduces the level of cartilage chondrocyte death in vivo in experimental dog osteoarthritis: inhibition of pro-apoptotic factors. J Rheumatol. 2002 Jul;29(7):1446-53. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

