Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0NH1E
|
|||
| Former ID |
DIB000651
|
|||
| Drug Name |
Org-231295
|
|||
| Synonyms |
3'-Carbamoyl-[1,1'-biphenyl]-3-yl cyclohexylcarbamate; 546141-08-6; URB597; URB-597; URB 597; FAAH Inhibitor II; KDS-4103; UNII-PX47LB88FO; 3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate; PX47LB88FO; CHEMBL184238; 3'-Carbamoylbiphenyl-3-yl cyclohexylcarbamate; [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate; Carbamic acid, N-cyclohexyl-, 3'-(aminocarbonyl)[1,1'-biphenyl]-3-yl ester; Cyclohexyl-carbamic acid 3'-carbamoyl-biphenyl-3-yl ester; KDS-1243; FAAH inhibitor, Merck & Co; FAAH inhibitor, Schering-Plough/Kadmus/university of California; 3'-carbamoylbiphenyl-3-yl cyclohexylcarbamate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anxiety disorder [ICD-11: 6B00-6B0Z; ICD-10: R45.0] | Investigative | [1] | |
| Company |
University of California
|
|||
| Structure |
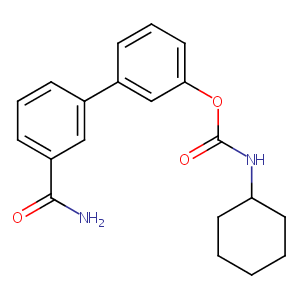 |
Download2D MOL |
||
| Formula |
C20H22N2O3
|
|||
| Canonical SMILES |
C1CCC(CC1)NC(=O)OC2=CC=CC(=C2)C3=CC(=CC=C3)C(=O)N
|
|||
| InChI |
1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24)
|
|||
| InChIKey |
ROFVXGGUISEHAM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 546141-08-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
1712159, 4117632, 6954439, 8844535, 14875581, 26758692, 29217673, 39816644, 47204541, 50076611, 50622803, 53790751, 53800779, 56365487, 57298451, 57409005, 89062649, 103455066, 103867351, 105221266, 110370747, 118265378, 124360783, 124582512, 125160794, 125164696, 125334103, 131318389, 134345885, 135246806, 135367488, 135683180, 136340244, 136349528, 136367659, 136373638, 137125269, 137189432, 139529511, 152258271, 152344299, 160647108, 162011484, 162035022, 162194395, 163125113, 163688151, 164194104, 164195801, 165437862
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fatty acid amide hydrolase (FAAH) | Target Info | Inhibitor | [2], [3] |
| BioCyc | Anandamide degradation | |||
| KEGG Pathway | Retrograde endocannabinoid signaling | |||
| Panther Pathway | Anandamide degradation | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4339). | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1400). | |||
| REF 3 | 1-Indol-1-yl-propan-2-ones and related heterocyclic compounds as dual inhibitors of cytosolic phospholipase A(2)alpha and fatty acid amide hydrolase. Bioorg Med Chem. 2010 Jan 15;18(2):945-52. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

