Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0NN8N
|
|||
| Former ID |
DNCL001691
|
|||
| Drug Name |
TNX-102
|
|||
| Synonyms |
Cyclobenzaprine very low dose
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Fibromyalgia [ICD-11: MG30.01; ICD-10: M79.7; ICD-9: 729.1] | Phase 3 | [1] | |
| Post-traumatic stress disorder [ICD-11: 6B40; ICD-10: F43.1, F62.0; ICD-9: 309.81] | Phase 3 | [2] | ||
| Company |
TONIX Pharmaceuticals
|
|||
| Structure |
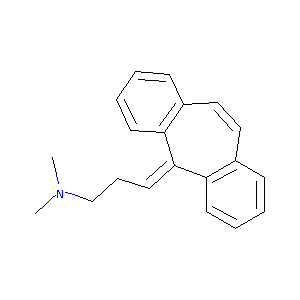 |
Download2D MOL |
||
| Formula |
C20H22ClN
|
|||
| Canonical SMILES |
CN(C)CCC=C1C2=CC=CC=C2C=CC3=CC=CC=C31.Cl
|
|||
| InChI |
1S/C20H21N.ClH/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20;/h3-6,8-14H,7,15H2,1-2H3;1H
|
|||
| InChIKey |
VXEAYBOGHINOKW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 6202-23-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
551139, 562883, 563490, 7847837, 8149930, 8166565, 10321738, 12015332, 14800994, 17404866, 24278315, 26612423, 26680154, 26748730, 26748731, 29289839, 49961820, 50106063, 50107452, 50107453, 50583809, 53777409, 56463037, 57331212, 91011923, 92124735, 92125456, 92303558, 92714414, 96079583, 103770619, 103914225, 104178963, 104356270, 118844966, 124637153, 124891634, 124891635, 124891636, 124891637, 125354670, 126624155, 126655420, 134987784, 136990691, 137263562, 143491823, 143491824, 144075566, 144115866
|
|||
| ChEBI ID |
CHEBI:3997
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides uniformis
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Bacteroides uniformis was decreased by Cyclobenzaprine hydrochloride (adjusted p-values: 4.35E-03). | |||
|
Studied Microbe: Bacteroides vulgatus
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Bacteroides vulgatus was decreased by Cyclobenzaprine hydrochloride (adjusted p-values: 5.98E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Veillonellales | ||||
|
Studied Microbe: Veillonella parvula
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Veillonella parvula was decreased by Cyclobenzaprine hydrochloride (adjusted p-values: 8.10E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Verrucomicrobiales | ||||
|
Studied Microbe: Akkermansia muciniphila
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Akkermansia muciniphila was decreased by Cyclobenzaprine hydrochloride (adjusted p-values: 1.35E-03). | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017946) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 4 | Clinical pipeline report, company report or official report of Tonix Pharmaceuticals. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

